One-Carbon Metabolism in Alzheimer's Disease and Parkinson's Disease Brain Tissue
- PMID: 35276958
- PMCID: PMC8838558
- DOI: 10.3390/nu14030599
One-Carbon Metabolism in Alzheimer's Disease and Parkinson's Disease Brain Tissue
Abstract
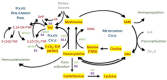
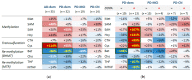
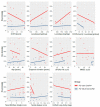
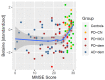
Disruptions in one-carbon metabolism and elevated homocysteine have been previously implicated in the development of dementia associated with Alzheimer's disease (AD) and Parkinson's disease (PD). Moreover, a PD diagnosis itself carries substantial risk for the development of dementia. This is the first study that explores alterations in one-carbon metabolism in AD and PD directly in the human brain frontal cortex, the primary center of cognition. Applying targeted liquid chromatography-tandem mass spectrometry (LC-MS/MS), we analyzed post-mortem samples obtained from 136 subjects (35 AD, 65 PD, 36 controls). We found changes in one-carbon metabolites that indicate inefficient activation of cystathionine β-synthase (CBS) in AD and PD subjects with dementia, the latter seemingly accompanied by a restricted re-methylation flow. Levodopa-carbidopa is known to reduce available vitamin B6, which would explain the hindered CBS activity. We present evidence of temporary non-protein-bound homocysteine accumulation upon levodopa intake in the brain of PD subjects with dementia but not in non-demented PD subjects. Importantly, this homocysteine elevation is not related to levodopa dosage, disease progression, or histopathological markers but exclusively to the dementia status. We hypothesize that this levodopa-induced effect is a direct cause of dementia in PD in susceptible subjects with reduced re-methylation capacity. Furthermore, we show that betaine best correlates with cognitive score even among PD subjects alone and discuss nutritional recommendations to improve one-carbon metabolism function.
Keywords: Alzheimer’s disease; Parkinson’s disease; betaine; brain frontal cortex; dementia; homocysteine; levodopa; metabolomics; one-carbon metabolism.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Zieminska E., Lazarewicz J.W. Excitotoxic neuronal injury in chronic homocysteine neurotoxicity studied in vitro: The role of NMDA and group I metabotropic glutamate receptors. Acta Neurobiol. Exp. Wars. 2006;66:301–309. - PubMed
-
- Graham I.M., Daly L.E., Refsum H.M., Robinson K., Brattström L.E., Ueland P.M., Palma-Reis R.J., Boers G.H., Sheahan R.G., Israelsson B., et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. JAMA. 1997;277:1775–1781. doi: 10.1001/jama.1997.03540460039030. - DOI - PubMed
-
- Virtanen J.K., Voutilainen S., Happonen P., Alfthan G., Kaikkonen J., Mursu J., Rissanen T.H., Kaplan G.A., Korhonen M.J., Sivenius J., et al. Serum homocysteine, folate and risk of stroke: Kuopio Ischaemic Heart Disease Risk Factor (KIHD) Study. Eur. J. Cardiovasc. Prev. Rehabil. 2005;12:369–375. doi: 10.1097/01.hjr.0000160834.75466.b0. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

