Probiotic Consumption Boosts Thymus in Obesity and Senescence Mouse Models
- PMID: 35276973
- PMCID: PMC8838891
- DOI: 10.3390/nu14030616
Probiotic Consumption Boosts Thymus in Obesity and Senescence Mouse Models
Abstract
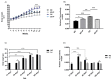
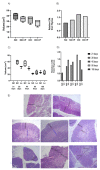
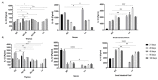
The ability of the immune system to respond to different pathogens throughout life requires the constant production and selection of T cells in the thymus. This immune organ is very sensitive to age, infectious processes and nutrition disorders (obesity and malnutrition). Several studies have shown that the incorporation of some probiotic bacteria or probiotic fermented milk in the diet has beneficial effects, not only at the intestinal level but also on distant mucosal tissues, improving the architecture of the thymus in a malnutrition model. The aim of the present study was to determine whether supplementation with the probiotic strain Lactobacillus casei CRL 431 and/or its cell wall could improve body weight, intestinal microbiota and thymus structure and function in both obese and aging mice. We evaluated probiotic administration to BALB/c mice in 2 experimental mouse models: obesity and senescence, including mice of different ages (21, 28, 45, 90 and 180 days). Changes in thymus size and histology were recorded. T-lymphocyte population and cytokine production were also determined. The consumption of probiotics improved the cortical/medullary ratio, the production and regulation of cytokines and the recovery of mature T-lymphocyte populations of the thymus in obese and old mice. Probiotic incorporation into the diet could not only modulate the immune system but also lead to thymus function recovery, thus improving quality of life.
Keywords: aging; immune system; obesity; probiotic cell wall; probiotics; thymus.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

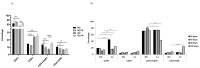


References
-
- Savino W., Dardenne M. Neuroendocrine Control of Thymus Physiology. Endocr. Rev. 2000;21:412–443. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

