Timing of Complementary Feeding, Growth, and Risk of Non-Communicable Diseases: Systematic Review and Meta-Analysis
- PMID: 35277061
- PMCID: PMC8840757
- DOI: 10.3390/nu14030702
Timing of Complementary Feeding, Growth, and Risk of Non-Communicable Diseases: Systematic Review and Meta-Analysis
Abstract
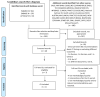
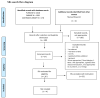
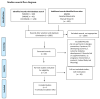
No consensus currently exists on the appropriate age for the introduction of complementary feeding (CF). In this paper, a systematic review is conducted that investigates the effects of starting CF in breastfed and formula-fed infants at 4, 4-6, or 6 months of age (i) on growth at 12 months of age, (ii) on the development of overweight/obesity at 3-6 years of age, (iii) on iron status, and (iv) on the risk of developing (later in life) type 2 diabetes mellitus (DM2) and hypertension. An extensive literature search identified seven studies that evaluated the effects of the introduction of CF at the ages in question. No statistically significant differences related to the age at which CF is started were observed in breastfed or formula-fed infants in terms of the following: iron status, weight, length, and body mass index Z-scores (zBMI) at 12 months, and development of overweight/obesity at 3 years. No studies were found specifically focused on the age range for CF introduction and risk of DM2 and hypertension. Introducing CF before 6 months in healthy term-born infants living in developed countries is essentially useless, as human milk (HM) and formulas are nutritionally adequate up to 6 months of age.
Keywords: breastfeeding; complementary feeding; early nutrition; formula feeding; growth; human milk; hypertension; obesity; overweight; type 2 diabetes mellitus (DM2); weaning.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures
References
-
- WHO. UNICEF . Global Strategy for Infant and Young Child Feeding. WHO; Geneva, Switzerland: 2003. [(accessed on 7 August 2020)]. Available online: www.who.int/nutrition/publications/infantfeeding/9241562218/en/index.html.
-
- Castenmiller J., de Henauw S., Hirsch-Ernst K.-I., Kearney J., Maciuk A., Mangelsdorf I., McArdle H.J., Naska A., Pelaez C., Pentieva K., et al. EFSA Panel on NDA—Scientific Opinion on the appropriate age range for introduction of complementary feeding into an infant’s diet. EFSA J. 2019;17:5780. - PMC - PubMed
-
- WHO . Guiding Principles for Feeding Non-Breastfed Children 6–24 Months of Age. WHO; Geneva, Switzerland: 2005. [(accessed on 7 August 2020)]. Available online: http://apps.who.int/iris/bitstream/10665/43281/1/9241593431.pdf?ua=1&ua=1.
-
- Fewtrell M., Bronsky J., Campoy C., Domello M., Embleton N., Fidler Mis N., Hojsak I., Hulst J.M., Indrio F., Lapillonne A., et al. Complementary feeding: A position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017;64:119–132. doi: 10.1097/MPG.0000000000001454. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical