Stroke Among SARS-CoV-2 Vaccine Recipients in Mexico: A Nationwide Descriptive Study
- PMID: 35277439
- PMCID: PMC9141628
- DOI: 10.1212/WNL.0000000000200388
Stroke Among SARS-CoV-2 Vaccine Recipients in Mexico: A Nationwide Descriptive Study
Abstract
Background and objectives: Information on stroke among severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines remains scarce. We report stroke incidence as an adverse event following immunization (AEFI) among recipients of 79,399,446 doses of 6 different SARS-CoV-2 vaccines (BNT162b2, ChAdOx1 nCov-19, Gam-COVID-Vac, CoronaVac, Ad5-nCoV, and Ad26.COV2-S) between December 24, 2020, and August 31, 2021, in Mexico.
Methods: This retrospective descriptive study analyzed stroke incidence per million doses among hospitalized adult patients (≥18 years) during an 8-month interval. According to the World Health Organization, AEFIs were defined as clinical events occurring within 30 days after immunization and categorized as either nonserious or serious, depending on severity, treatment, and hospital admission requirements. Acute ischemic stroke (AIS), intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), and cerebral venous thrombosis (CVT) cases were collected through a passive epidemiologic surveillance system in which local health providers report potential AEFI to the Mexican General Board of Epidemiology. Data were captured with standardized case report formats by an ad hoc committee appointed by the Mexican Ministry of Health to evaluate potential neurologic AEFI against SARS-COV-2.
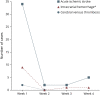
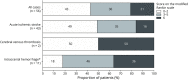
Results: We included 56 patients (31 female patients [55.5%]) for an overall incidence of 0.71 cases per 1,000,000 administered doses (95% CI 0.54-0.92). Median age was 65 years (interquartile range [IQR] 55-76 years); median time from vaccination to stroke (of any subtype) was 2 days (IQR 1-5 days). In 27 (48.2%) patients, the event was diagnosed within the first 24 hours after immunization. The most frequent subtype was AIS in 43 patients (75%; 0.54 per 1,000,000 doses, 95% CI 0.40-0.73), followed by ICH in 9 (16.1%; 0.11 per 1,000,000 doses, 95% CI 0.06-0.22) and SAH and CVT, each with 2 cases (3.6%; 0.03 per 1,000,000 doses, 95% CI 0.01-0.09). Overall, the most common risk factors were hypertension in 33 (58.9%) patients and diabetes in 22 (39.3%). Median hospital length of stay was 6 days (IQR 4-13 days). At discharge, functional outcome was good (modified Rankin Scale score 0-2) in 41.1% of patients; in-hospital mortality rate was 21.4%.
Discussion: Stroke is an exceedingly rare AEFI against SARS-CoV-2. Preexisting stroke risk factors were identified in most patients. Further research is needed to evaluate causal associations between SARS-COV-2 vaccines and stroke.
© 2022 American Academy of Neurology.
Figures

Comment in
-
Reader Response: Stroke Among SARS-CoV-2 Vaccine Recipients in Mexico: A Nationwide Descriptive Study.Neurology. 2022 Oct 11;99(15):673-674. doi: 10.1212/WNL.0000000000201310. Neurology. 2022. PMID: 36216519 No abstract available.
-
Reader Response: Stroke Among SARS-CoV-2 Vaccine Recipients in Mexico: A Nationwide Descriptive Study.Neurology. 2022 Oct 11;99(15):672. doi: 10.1212/WNL.0000000000201308. Neurology. 2022. PMID: 36216521 No abstract available.
-
Author Response: Stroke Among SARS-CoV-2 Vaccine Recipients in Mexico: A Nationwide Descriptive Study.Neurology. 2022 Oct 11;99(15):673. doi: 10.1212/WNL.0000000000201309. Neurology. 2022. PMID: 36216526 No abstract available.
-
Author Response: Stroke Among SARS-CoV-2 Vaccine Recipients in Mexico: A Nationwide Descriptive Study.Neurology. 2022 Oct 11;99(15):674. doi: 10.1212/WNL.0000000000201311. Neurology. 2022. PMID: 36216528 No abstract available.
References
-
- Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021;27(2):205-211. - PubMed
-
- Subsecretaría de Prevención y Promoción de la Salud, Instituto Nacional de Salut Pública. Política nacional rectora de vacunación contra el SARS-CoV-2 para la prevención de la COVID-19 en México. Accessed August 20, 2021. vacunacovid.gob.mx/wordpress/wp-content/uploads/2021/09/2021.09.28-PNVx_...
-
- Walter U, Fuchs M, Grossmann A, et al. Adenovirus-vectored COVID-19 vaccine–induced immune thrombosis of carotid artery: a case report. Neurology. 2021;97(15):716-719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
