Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes
- PMID: 35277453
- PMCID: PMC9185832
- DOI: 10.1136/gutjnl-2021-326264
Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes
Abstract
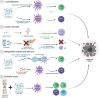
Despite the promising advances in novel cancer therapy such as immune checkpoint inhibitors (ICIs), limitations including therapeutic resistance and toxicity remain. In recent years, the relationship between gut microbiota and cancer has been extensively studied. Accumulating evidence reveals the role of microbiota in defining cancer therapeutic efficacy and toxicity. Unlike host genetics, microbiota can be easily modified via multiple strategies, including faecal microbiota transplantation (FMT), probiotics and antibiotics. Preclinical studies have identified the mechanisms on how microbes influence cancer treatment outcomes. Clinical trials have also demonstrated the potential of microbiota modulation in cancer treatments. Herein, we review the mechanistic insights of gut microbial interactions with chemotherapy and ICIs, particularly focusing on the interplay between gut bacteria and the pharmacokinetics (eg, metabolism, enzymatic degradation) or pharmacodynamics (eg, immunomodulation) of cancer treatment. The translational potential of basic findings in clinical settings is then explored, including using microbes as predictive biomarkers and microbial modulation by antibiotics, probiotics, prebiotics, dietary modulations and FMT. We further discuss the current limitations of gut microbiota modulation in patients with cancer and suggest essential directions for future study. In the era of personalised medicine, it is crucial to understand the microbiota and its interactions with cancer. Manipulating the gut microbiota to augment cancer therapeutic responses can provide new insights into cancer treatment.
Keywords: cancer; chemotherapy; enteric bacterial microflora; immunotherapy.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
