Smartphone detection of atrial fibrillation using photoplethysmography: a systematic review and meta-analysis
- PMID: 35277454
- PMCID: PMC9554073
- DOI: 10.1136/heartjnl-2021-320417
Smartphone detection of atrial fibrillation using photoplethysmography: a systematic review and meta-analysis
Abstract
Objectives: Timely diagnosis of atrial fibrillation (AF) is essential to reduce complications from this increasingly common condition. We sought to assess the diagnostic accuracy of smartphone camera photoplethysmography (PPG) compared with conventional electrocardiogram (ECG) for AF detection.
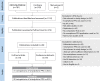
Methods: This is a systematic review of MEDLINE, EMBASE and Cochrane (1980-December 2020), including any study or abstract, where smartphone PPG was compared with a reference ECG (1, 3 or 12-lead). Random effects meta-analysis was performed to pool sensitivity/specificity and identify publication bias, with study quality assessed using the QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies-2) risk of bias tool.
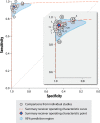
Results: 28 studies were included (10 full-text publications and 18 abstracts), providing 31 comparisons of smartphone PPG versus ECG for AF detection. 11 404 participants were included (2950 in AF), with most studies being small and based in secondary care. Sensitivity and specificity for AF detection were high, ranging from 81% to 100%, and from 85% to 100%, respectively. 20 comparisons from 17 studies were meta-analysed, including 6891 participants (2299 with AF); the pooled sensitivity was 94% (95% CI 92% to 95%) and specificity 97% (96%-98%), with substantial heterogeneity (p<0.01). Studies were of poor quality overall and none met all the QUADAS-2 criteria, with particular issues regarding selection bias and the potential for publication bias.
Conclusion: PPG provides a non-invasive, patient-led screening tool for AF. However, current evidence is limited to small, biased, low-quality studies with unrealistically high sensitivity and specificity. Further studies are needed, preferably independent from manufacturers, in order to advise clinicians on the true value of PPG technology for AF detection.
Keywords: atrial fibrillation; photoplethysmography; smartphone.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Dr Gill reports funding through the BigData@Heart Innovative Medicines Initiative, grant no.116074. Dr Bunting reports a grant from the University of Birmingham’s British Heart Foundation Accelerator Award (BHF AA/18/2/34218). Dr Sartini reports that Bayer AG is one of the partners that have funded the IMI framework and is employed by Bayer AG in this IMI collaboration. Mr Roth Cardoso has nothing to disclose. Ms Narges Ghoreishi has nothing to disclose. Dr Uh reports grants and personal fees from Innovative Medicines Initiative 2 BigData@Heart, grant no. 116074, during the conduct of the study. Dr Williams has nothing to disclose. Dr Kiliana Suzart-Woischnik has nothing to disclose. Dr Banerjee reports grants from AstraZeneca, outside the submitted work. Professor Asselbergs reports grants from Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No (116074), during the conduct of the study and is supported by UCL Hospitals NIHR Biomedical Research Centre. Professor MJC Eijkemans has nothing to disclose. Professor Gkoutos has nothing to disclose. Professor Kotecha reports grants from the National Institute for Health Research (NIHR CDF-2015-08-074 RATE-AF; NIHR HTA-130280 DaRe2THINK; NIHR EME-132974 D2T-NV), the British Heart Foundation (PG/17/55/33087 and AA/18/2/34218), EU/EFPIA Innovative Medicines Initiative (BigData@Heart 116074), the European Society of Cardiology supported by educational grants from Boehringer Ingelheim/BMS-Pfizer Alliance/Bayer/Daiichi Sankyo/Boston Scientific, the NIHR/University of Oxford Biomedical Research Centre and British Heart Foundation/University of Birmingham Accelerator Award (STEEER-AF NCT04396418), Amomed Pharma and IRCCS San Raffaele/Menarini (Beta-blockers in Heart Failure Collaborative Group NCT0083244); in addition to personal fees from Bayer (Advisory Board), AtriCure (Speaker fees), Protherics Medicines Development (Advisory Board) and Myokardia (Advisory Board).
Figures


References
-
- Hindricks G, Potpara T, Dagres N. Esc guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for Cardio-Thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J 2020;2021:373–498. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
