Cortical thinning over two years after first-episode psychosis depends on age of onset
- PMID: 35277520
- PMCID: PMC8917180
- DOI: 10.1038/s41537-021-00196-7
Cortical thinning over two years after first-episode psychosis depends on age of onset
Abstract
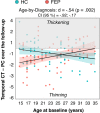
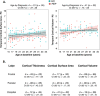
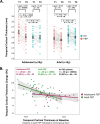
First-episode psychosis (FEP) patients show structural brain abnormalities at the first episode. Whether the cortical changes that follow a FEP are progressive and whether age at onset modulates these changes remains unclear. This is a multicenter MRI study in a deeply phenotyped sample of 74 FEP patients with a wide age range at onset (15-35 years) and 64 neurotypical healthy controls (HC). All participants underwent two MRI scans with a 2-year follow-up interval. We computed the longitudinal percentage of change (PC) for cortical thickness (CT), surface area (CSA) and volume (CV) for frontal, temporal, parietal and occipital lobes. We used general linear models to assess group differences in PC as a function of age at FEP. We conducted post-hoc analyses for metrics where PC differed as a function of age at onset. We found a significant age-by-diagnosis interaction effect for PC of temporal lobe CT (d = 0.54; p = 002). In a post-hoc-analysis, adolescent-onset (≤19 y) FEP showed more severe longitudinal cortical thinning in the temporal lobe than adolescent HC. We did not find this difference in adult-onset FEP compared to adult HC. Our study suggests that, in individuals with psychosis, CT changes that follow the FEP are dependent on the age at first episode, with those with an earlier onset showing more pronounced cortical thinning in the temporal lobe.
© 2022. The Author(s).
Conflict of interest statement
L.P-C. has received grant support from Fundación Alicia Koplowitz and Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation, and has received honoraria or grants unrelated to the present work from Rubió, Rovi and Janssen. C.D-C. holds a Juan Rodés grant from Instituto de Salud Carlos III (JR19/00024), Spanish Ministry of Science and Innovation, and has received honoraria from AbbVie, Sanofi, and Exeltis. C.M. has received grants and served as consultant or advisor from European Union Funds, Fundación Alicia Koplowitz, Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation, CIBERSAM, Janssen, Angelini, Servier, Nuvelution, Otsuka, Lundbeck and Esteve. E.V. has received grants and served as consultant, advisor or CME speaker unrelated to the present work for the following entities: AB-Biotics, Abbott, Allergan, Angelini, Dainippon Sumitomo Pharma, Ferrer, Gedeon Richter, Janssen, Lundbeck, Otsuka, Sage, Sanofi-Aventis, Sunovion, and Takeda. C.A. has been a consultant, to or has received honoraria or grants from Acadia, Angelini, Gedeon Richter, Janssen Cilag, Lundbeck, Minerva, Otsuka, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion, and Takeda. D.F. has been a consultant to and/or receiving fees from Angelini, Eisai, IE4Lab, Janssen, Lundbeck, and Otsuka and has received grant support from Fundación Alicia Koplowitz and Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation. M.B. has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory board of ABBiotics, Adamed, Angelini, Casen Recordati, Janssen-Cilag, Menarini, Rovi and Takeda. M.P. has received grants and served as consultant, advisor or CME speaker unrelated to the present work for the following entities: Servier, Exceltis, Lundbeck, Fundación Alicia Koplowitz, Fundación Familia Alonso, Ministry of Health, and Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation. The remaining authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

