A computational study of red blood cell deformability effect on hemodynamic alteration in capillary vessel networks
- PMID: 35277592
- PMCID: PMC8917159
- DOI: 10.1038/s41598-022-08357-z
A computational study of red blood cell deformability effect on hemodynamic alteration in capillary vessel networks
Abstract
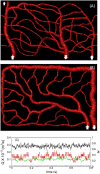
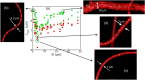
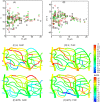
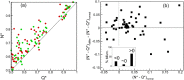
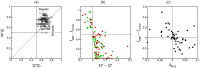
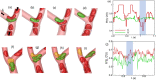
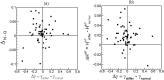
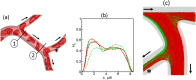
Capillary blood vessels, the smallest vessels in the body, form an intricate network with constantly bifurcating, merging and winding vessels. Red blood cells (RBCs) must navigate through such complex microvascular networks in order to maintain tissue perfusion and oxygenation. Normal, healthy RBCs are extremely deformable and able to easily flow through narrow vessels. However, RBC deformability is reduced in many pathological conditions and during blood storage. The influence of reduced cell deformability on microvascular hemodynamics is not well established. Here we use a high-fidelity, 3D computational model of blood flow that retains exact geometric details of physiologically realistic microvascular networks, and deformation of every one of nearly a thousand RBCs flowing through the networks. We predict that reduced RBC deformability alters RBC trafficking with significant and heterogeneous changes in hematocrit. We quantify such changes along with RBC partitioning and lingering at vascular bifurcations, perfusion and vascular resistance, and wall shear stress. We elucidate the cellular-scale mechanisms that cause such changes. We show that such changes arise primarily due to the altered RBC dynamics at vascular bifurcations, as well as cross-stream migration. Less deformable cells tend to linger less at majority of bifurcations increasing the fraction of RBCs entering the higher flow branches. Changes in vascular resistance also seen to be heterogeneous and correlate with hematocrit changes. Furthermore, alteration in RBC dynamics is shown to cause localized changes in wall shear stress within vessels and near vascular bifurcations. Such heterogeneous and focal changes in hemodynamics may be the cause of morphological abnormalities in capillary vessel networks as observed in several diseases.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Beare JE, Curtis-Whitchurch L, LeBlanc AJ, Hoying JB. Microvasculature in health and disease. In: Vasan RS, Sawyer DB, editors. Encyclopedia of Cardiovascular Research and Medicine. Elsevier; 2018. pp. 349–364.
-
- Couturier A, Mane V, Bonnin S, Erginay A, Massin P, et al. Capillary plexus anomalies in diabetic retinopathy on optical coherence tomography angiography. Retina. 2015;35:2384–3239. - PubMed
-
- Cheung ATW, Chen PCY, Larkin EC, Duong PL, Ramanujan S, et al. Microvascular abnormalities in sickle cell disease: A computer-assisted intravital microscopy study. Blood. 2002;99:3999–4005. - PubMed
-
- Cheung CY, Zheng Y, Hsu W, Lee ML, Lau QP, et al. Retinal vascular tortuosity, blood pressure, and cardiovascular risk factors. Ophthalmology. 2011;118:812–818. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

