Factors associated with dexamethasone efficacy in COVID-19. A retrospective investigative cohort study
- PMID: 35277862
- PMCID: PMC9088322
- DOI: 10.1002/jmv.27712
Factors associated with dexamethasone efficacy in COVID-19. A retrospective investigative cohort study
Abstract
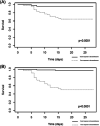
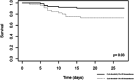
Dexamethasone has demonstrated efficacy in reducing mortality in COVID-19. However, its practical use is badly defined. We aimed to investigate factors associated with dexamethasone efficacy in real life. Our retrospective study was conducted in two university hospitals between September and November 2020 and included all the consecutive hospitalized patients with a laboratory-confirmed SARS-CoV-2 infection assessed by RT-PCR, treated with intravenous dexamethasone (6 mg/day). Among 111 patients, 10.6% necessitated a transfer into the intensive care unit (ICU) and the 28-day mortality rate was 17.1%. The 28-day mortality rate was significantly lower in patients who demonstrated improvement at 48 h (hazard ratio [HR]: 0.17, 95% confidence interval [CI]: 0.04-0.78, p = 0.02) and 96 h (HR: 0.07, 95% CI: 0.02-0.31, p = 0.0005) after dexamethasone initiation. Apart from well-known risk factors (age, hypertension, active cancer, severe lesions on chest computed tomography [CT] scan), we found that a high viral load in nasopharyngeal swab (Cycle threshold <30) at dexamethasone initiation was associated with higher 28-day mortality (66.6% vs. 36.7%, p = 0.03). Patients who did not receive antibiotics at dexamethasone initiation had a higher rate of transfer into the ICU (55.6% vs. 23.5%, p = 0.045) with a trend towards higher mortality in case of severe or critical lesions on CT scan (75.0% vs. 25.0%, p = 0.053). Patients who did not improve within 2-4 days after steroid initiation have a bad prognosis and should receive additional anti-inflammatory drugs. Our data suggest better efficacy of dexamethasone in patients with a low or negative viral load, receiving broad-spectrum antibiotics.
Keywords: COVID-19; antibiotics; dexamethasone; intensive care unit; mortality.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- WHO Director‐General's remarks at the media briefing on 2019‐nCoV on 11 February 2020., Accessed February 3 2021. https://www.who.int/director-general/speeches/detail/who-director-genera...
-
- WHO Coronavirus (COVID‐19) Dashboard., Accessed April 25 2021. https://covid19.who.int
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

