Survival After Induction Chemotherapy and Chemoradiation Versus Chemoradiation and Adjuvant Chemotherapy for Locally Advanced Rectal Cancer
- PMID: 35278070
- PMCID: PMC9074984
- DOI: 10.1093/oncolo/oyac025
Survival After Induction Chemotherapy and Chemoradiation Versus Chemoradiation and Adjuvant Chemotherapy for Locally Advanced Rectal Cancer
Abstract
Background: Total neoadjuvant therapy (TNT) improves tumor response in locally advanced rectal cancer (LARC) patients compared to neoadjuvant chemoradiotherapy alone. The effect of TNT on patient survival has not been fully investigated.
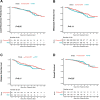
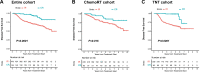
Materials and methods: This was a retrospective case series of patients with LARC at a comprehensive cancer center. Three hundred and eleven patients received chemoradiotherapy (chemoRT) as the sole neoadjuvant treatment and planned adjuvant chemotherapy, and 313 received TNT (induction fluorouracil and oxaliplatin-based chemotherapy followed by chemoradiotherapy in the neoadjuvant setting). These patients then underwent total mesorectal excision or were entered in a watch-and-wait protocol. The proportion of patients with complete response (CR) after neoadjuvant therapy (defined as pathological CR or clinical CR sustained for 2 years) was compared by the χ2 test. Disease-free survival (DFS), local recurrence-free survival, distant metastasis-free survival, and overall survival were assessed by Kaplan-Meier analysis and log-rank test. Cox regression models were used to further evaluate DFS.
Results: The rate of CR was 20% for chemoRT and 27% for TNT (P=.05). DFS, local recurrence-free survival, metastasis-free survival, and overall survival were no different. Disease-free survival was not associated with the type of neoadjuvant treatment (hazard ratio [HR] 1.3; 95% confidence interval [CI] 0.93-1.80; P = .12).
Conclusions: Although TNT does not prolong survival than neoadjuvant chemoradiotherapy plus intended postoperative chemotherapy, the higher response rate associated with TNT may create opportunities to preserve the rectum in more patients with LARC.
Keywords: Total neoadjuvant therapy; locally advanced rectal cancer; response; survival.
© The Author(s) 2022. Published by Oxford University Press.
Figures
References
-
- Sauer R, Liersch T, Merkel S, et al. . Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926-1933. 10.1200/JCO.2011.40.1836 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

