Mechanically Induced Nuclear Shuttling of β-Catenin Requires Co-transfer of Actin
- PMID: 35278073
- PMCID: PMC9633329
- DOI: 10.1093/stmcls/sxac006
Mechanically Induced Nuclear Shuttling of β-Catenin Requires Co-transfer of Actin
Abstract
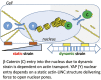
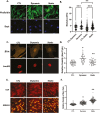
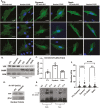
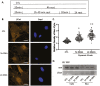
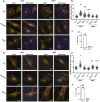
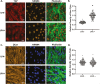
Mesenchymal stem cells (MSCs) respond to environmental forces with both cytoskeletal re-structuring and activation of protein chaperones of mechanical information, β-catenin, and yes-associated protein 1 (YAP1). To function, MSCs must differentiate between dynamic forces such as cyclic strains of extracellular matrix due to physical activity and static strains due to ECM stiffening. To delineate how MSCs recognize and respond differently to both force types, we compared effects of dynamic (200 cycles × 2%) and static (1 × 2% hold) strain on nuclear translocation of β-catenin and YAP1 at 3 hours after force application. Dynamic strain induced nuclear accumulation of β-catenin, and increased cytoskeletal actin structure and cell stiffness, but had no effect on nuclear YAP1 levels. Critically, both nuclear actin and nuclear stiffness increased along with dynamic strain-induced β-catenin transport. Augmentation of cytoskeletal structure using either static strain or lysophosphatidic acid did not increase nuclear content of β-catenin or actin, but induced robust nuclear increase in YAP1. As actin binds β-catenin, we considered whether β-catenin, which lacks a nuclear localization signal, was dependent on actin to gain entry to the nucleus. Knockdown of cofilin-1 (Cfl1) or importin-9 (Ipo9), which co-mediate nuclear transfer of G-actin, prevented dynamic strain-mediated nuclear transfer of both β-catenin and actin. In sum, dynamic strain induction of actin re-structuring promotes nuclear transport of G-actin, concurrently supporting nuclear access of β-catenin via mechanisms used for actin transport. Thus, dynamic and static strain activate alternative mechanoresponses reflected by differences in the cellular distributions of actin, β-catenin, and YAP1.
Keywords: YAP-signaling protein; actin cytoskeleton; mechanical stress; mesenchymal stem cell; nuclear transport.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

