Modeling the Prognostic Impact of Circulating Tumor Cells Enumeration in Metastatic Breast Cancer for Clinical Trial Design Simulation
- PMID: 35278078
- PMCID: PMC9255982
- DOI: 10.1093/oncolo/oyac045
Modeling the Prognostic Impact of Circulating Tumor Cells Enumeration in Metastatic Breast Cancer for Clinical Trial Design Simulation
Abstract
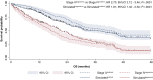
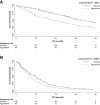
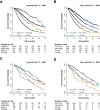
Despite the strong prognostic stratification of circulating tumor cells (CTCs) enumeration in metastatic breast cancer (MBC), current clinical trials usually do not include a baseline CTCs in their design. This study aimed to generate a classifier for CTCs prognostic simulation in existing datasets for hypothesis generation in patients with MBC. A K-nearest neighbor machine learning algorithm was trained on a pooled dataset comprising 2436 individual MBC patients from the European Pooled Analysis Consortium and the MD Anderson Cancer Center to identify patients likely to have CTCs ≥ 5/7 mL blood (StageIVaggressive vs StageIVindolent). The model had a 65.1% accuracy and its prognostic impact resulted in a hazard ratio (HR) of 1.89 (Simulatedaggressive vs SimulatedindolentP < .001), similar to patients with actual CTCs enumeration (HR 2.76; P < .001). The classifier's performance was then tested on an independent retrospective database comprising 446 consecutive hormone receptor (HR)-positive HER2-negative MBC patients. The model further stratified clinical subgroups usually considered prognostically homogeneous such as patients with bone-only or liver metastases. Bone-only disease classified as Simulatedaggressive had a significantly worse overall survival (OS; P < .0001), while patients with liver metastases classified as Simulatedindolent had a significantly better prognosis (P < .0001). Consistent results were observed for patients who had undergone CTCs enumeration in the pooled population. The differential prognostic impact of endocrine- (ET) and chemotherapy (CT) was explored across the simulated subgroups. No significant differences were observed between ET and CT in the overall population, both in terms of progression-free survival (PFS) and OS. In contrast, a statistically significant difference, favoring CT over ET was observed among Simulatedaggressive patients (HR: 0.62; P = .030 and HR: 0.60; P = .037, respectively, for PFS and OS).
Keywords: K-nearest neighbor; biomarker; clinical trial model; liquid biopsy; machine learning.
© The Author(s) 2022. Published by Oxford University Press.
Figures


References
-
- Kiely BE, Soon YY, Tattersall MHN, Stockler MR.. How long have I got? Estimating typical, best-case, and worst-case scenarios for patients starting first-line chemotherapy for metastatic breast cancer: a systematic review of recent randomized trials. J Clin Oncol. 2011;29(4):456-463. 10.1200/JCO.2010.30.2174. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

