Determining the lower limit of detection required for HCV viral load assay for test of cure following direct-acting antiviral-based treatment regimens: Evidence from a global data set
- PMID: 35278339
- PMCID: PMC9248016
- DOI: 10.1111/jvh.13672
Determining the lower limit of detection required for HCV viral load assay for test of cure following direct-acting antiviral-based treatment regimens: Evidence from a global data set
Abstract
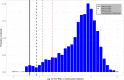
Achieving global elimination of hepatitis C virus requires a substantial scale-up of testing. Point-of-care HCV viral load assays are available as an alternative to laboratory-based assays to promote access in hard to reach or marginalized populations. The diagnostic performance and lower limit of detection are important attributes of these new assays for both diagnosis and test of cure. Therefore, our objective was to determine an acceptable LLoD for detectable HCV viraemia as a test for cure, 12 weeks post-treatment (SVR12). We assembled a global data set of patients with detectable viraemia at SVR12 from observational databases from 9 countries (Egypt, the United States, United Kingdom, Georgia, Ukraine, Myanmar, Cambodia, Pakistan, Mozambique) and two pharmaceutical-sponsored clinical trial registries. We examined the distribution of HCV viral load at SVR12 and presented the 90th, 95th, 97th and 99th percentiles. We used logistic regression to assess characteristics associated with low-level virological treatment failure (defined as <1000 IU/mL). There were 5973 cases of detectable viraemia at SVR12 from the combined data set. Median detectable HCV RNA at SVR12 was 287,986 IU/mL. The level of detection for the 95th percentile was 227 IU/mL (95% CI 170-276). Females and those with minimal fibrosis were more likely to experience low-level viraemia at SVR12 compared to men (adjusted odds ratio AOR = 1.60 95% confidence interval [CI] 1.30-1.97 and those with cirrhosis (AOR = 1.49 95% CI 1.15-1.93). In conclusion, an assay with a level of detection of 1000 IU/mL or greater may miss a proportion of those with low-level treatment failure.
Keywords: HCV; diagnostics; limit of detection; point-of-care testing.
© 2022 The Authors. Journal of Viral Hepatitis published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors report no other funding or conflicts of interest.
Figures
References
-
- World Health Organization . Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. 2021. https://www.who.int/publications/i/item/9789240027077
-
- World Health Organization . Global health sector streategy on viral hepatitis 2016‐2021: towards ending viral hepatitis. http://apps.who.int/iris/bitstream/10665/246177/1/WHO‐HIV‐2016.06‐eng.pd...

