3D-organoid culture supports differentiation of human CAR+ iPSCs into highly functional CAR T cells
- PMID: 35278370
- PMCID: PMC9119152
- DOI: 10.1016/j.stem.2022.02.009
3D-organoid culture supports differentiation of human CAR+ iPSCs into highly functional CAR T cells
Erratum in
-
3D-organoid culture supports differentiation of human CAR+ iPSCs into highly functional CAR T cells.Cell Stem Cell. 2022 Apr 7;29(4):651-653. doi: 10.1016/j.stem.2022.03.007. Cell Stem Cell. 2022. PMID: 35395190 No abstract available.
Abstract
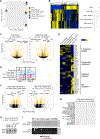
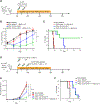
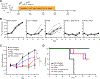
Unlimited generation of chimeric antigen receptor (CAR) T cells from human-induced pluripotent stem cells (iPSCs) is an attractive approach for "off-the-shelf" CAR T cell immunotherapy. Approaches to efficiently differentiate iPSCs into canonical αβ T cell lineages, while maintaining CAR expression and functionality, however, have been challenging. We report that iPSCs reprogramed from CD62L+ naive and memory T cells followed by CD19-CAR engineering and 3D-organoid system differentiation confers products with conventional CD8αβ-positive CAR T cell characteristics. Expanded iPSC CD19-CAR T cells showed comparable antigen-specific activation, degranulation, cytotoxicity, and cytokine secretion compared with conventional CD19-CAR T cells and maintained homogeneous expression of the TCR derived from the initial clone. iPSC CD19-CAR T cells also mediated potent antitumor activity in vivo, prolonging survival of mice with CD19+ human tumor xenografts. Our study establishes feasible methodologies to generate highly functional CAR T cells from iPSCs to support the development of "off-the-shelf" manufacturing strategies.
Keywords: 3D-organoid culture; CAR; PSC-ATO; chimeric antigen receptor T cells; human iPSC; immunotherapy; off-the-shelf; pluripotent stem cell-artificial thymic organoid culture.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.E.B., S.J.F., and Z.W. are listed on patent(s) relating to this work: Forman, S.J., Brown, C.E., and Wang, Z. Generation of chimeric antigen receptor modified T cells from stem cells and therapeutic uses thereof. U.S. application n. 62/931125. PCT application no. PCT/US2020/059216. A.M.H., C.S.S., and G.M.C. are co-founders of Pluto Immunotherapeutics, which holds certain rights over intellectual property relating to the ATO system.
Figures


Comment in
-
Successful organoid-mediated generation of iPSC-derived CAR-T cells.Cell Stem Cell. 2022 Apr 7;29(4):493-495. doi: 10.1016/j.stem.2022.03.005. Cell Stem Cell. 2022. PMID: 35395182
Similar articles
-
Induced Pluripotent Stem Cells (iPSCs) Provide a Potentially Unlimited T Cell Source for CAR-T Cell Development and Off-the-Shelf Products.Pharm Res. 2021 Jun;38(6):931-945. doi: 10.1007/s11095-021-03067-z. Epub 2021 Jun 10. Pharm Res. 2021. PMID: 34114161 Review.
-
Successful organoid-mediated generation of iPSC-derived CAR-T cells.Cell Stem Cell. 2022 Apr 7;29(4):493-495. doi: 10.1016/j.stem.2022.03.005. Cell Stem Cell. 2022. PMID: 35395182
-
Induced Pluripotent Stem Cell-Derived Chimeric Antigen Receptor T Cells: The Intersection of Stem Cells and Immunotherapy.Cell Reprogram. 2023 Oct;25(5):195-211. doi: 10.1089/cell.2023.0041. Epub 2023 Sep 29. Cell Reprogram. 2023. PMID: 37782910 Review.
-
Generation and Functional Characterization of Anti-CD19 Chimeric Antigen Receptor-Natural Killer Cells from Human Induced Pluripotent Stem Cells.Int J Mol Sci. 2023 Jun 22;24(13):10508. doi: 10.3390/ijms241310508. Int J Mol Sci. 2023. PMID: 37445684 Free PMC article.
-
Human anti-PSCA CAR macrophages possess potent antitumor activity against pancreatic cancer.Cell Stem Cell. 2024 Jun 6;31(6):803-817.e6. doi: 10.1016/j.stem.2024.03.018. Epub 2024 Apr 24. Cell Stem Cell. 2024. PMID: 38663406 Free PMC article.
Cited by
-
'Off the shelf' immunotherapies: Generation and application of pluripotent stem cell-derived immune cells.Cell Prolif. 2023 Apr;56(4):e13425. doi: 10.1111/cpr.13425. Epub 2023 Mar 1. Cell Prolif. 2023. PMID: 36855955 Free PMC article. Review.
-
Allogeneic chimeric antigen receptor cell therapies for cancer: progress made and remaining roadblocks.Nat Rev Clin Oncol. 2025 Jan;22(1):10-27. doi: 10.1038/s41571-024-00959-y. Epub 2024 Nov 15. Nat Rev Clin Oncol. 2025. PMID: 39548270 Review.
-
Pluripotent stem cell-based immunotherapy: advances in translational research, cell differentiation, and gene modifications.Life Med. 2025 Jan 18;4(1):lnaf002. doi: 10.1093/lifemedi/lnaf002. eCollection 2025 Feb. Life Med. 2025. PMID: 40110110 Free PMC article. Review.
-
Strength of CAR signaling determines T cell versus ILC differentiation from pluripotent stem cells.Cell Rep. 2023 Mar 28;42(3):112241. doi: 10.1016/j.celrep.2023.112241. Epub 2023 Mar 11. Cell Rep. 2023. PMID: 36906850 Free PMC article.
-
Generation of allogeneic CAR-NKT cells from hematopoietic stem and progenitor cells using a clinically guided culture method.Nat Biotechnol. 2025 Mar;43(3):329-344. doi: 10.1038/s41587-024-02226-y. Epub 2024 May 14. Nat Biotechnol. 2025. PMID: 38744947 Free PMC article.
References
-
- Akbar AN, and Henson SM (2011). Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nature reviews Immunology 11, 289–295. - PubMed
-
- Black J, and Vos JM (2002). Establishment of an oriP/EBNA1-based episomal vector transcribing human genomic beta-globin in cultured murine fibroblasts. Gene Ther 9, 1447–1454. - PubMed
-
- Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, BorquezOjeda O, et al. (2011). Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118, 4817–4828. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

