The role of lipid second messengers in aldosterone synthesis and secretion
- PMID: 35278411
- PMCID: PMC9020094
- DOI: 10.1016/j.jlr.2022.100191
The role of lipid second messengers in aldosterone synthesis and secretion
Abstract
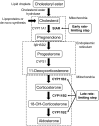
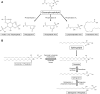
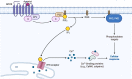
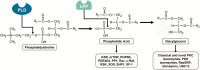
Second messengers are small rapidly diffusing molecules or ions that relay signals between receptors and effector proteins to produce a physiological effect. Lipid messengers constitute one of the four major classes of second messengers. The hydrolysis of two main classes of lipids, glycerophospholipids and sphingolipids, generate parallel profiles of lipid second messengers: phosphatidic acid (PA), diacylglycerol (DAG), and lysophosphatidic acid versus ceramide, ceramide-1-phosphate, sphingosine, and sphingosine-1-phosphate, respectively. In this review, we examine the mechanisms by which these lipid second messengers modulate aldosterone production at multiple levels. Aldosterone is a mineralocorticoid hormone responsible for maintaining fluid volume, electrolyte balance, and blood pressure homeostasis. Primary aldosteronism is a frequent endocrine cause of secondary hypertension. A thorough understanding of the signaling events regulating aldosterone biosynthesis may lead to the identification of novel therapeutic targets. The cumulative evidence in this literature emphasizes the critical roles of PA, DAG, and sphingolipid metabolites in aldosterone synthesis and secretion. However, it also highlights the gaps in our knowledge, such as the preference for phospholipase D-generated PA or DAG, as well as the need for further investigation to elucidate the precise mechanisms by which these lipid second messengers regulate optimal aldosterone production.
Keywords: adrenal cortex; glycerophospholipids; intracellular signaling; phospholipases; primary aldosteronism; signal transduction; sphingolipids; steroidogenesis.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest No author has an actual or perceived conflict of interest with the content of this article.
Figures
Similar articles
-
Sphingoid bases and phospholipase D activation.Chem Phys Lipids. 1996 May 24;80(1-2):27-36. doi: 10.1016/0009-3084(96)02543-1. Chem Phys Lipids. 1996. PMID: 8681426 Review.
-
Diacylglycerol, phosphatidic acid, and their metabolic enzymes in synaptic vesicle recycling.Adv Biol Regul. 2015 Jan;57:147-52. doi: 10.1016/j.jbior.2014.09.010. Epub 2014 Sep 28. Adv Biol Regul. 2015. PMID: 25446883 Free PMC article. Review.
-
Contribution of lipid second messengers to the regulation of phosphatidylcholine synthesis during cell cycle re-entry.Biochim Biophys Acta. 2004 Nov 8;1686(1-2):85-99. doi: 10.1016/j.bbalip.2004.09.001. Biochim Biophys Acta. 2004. PMID: 15522825
-
Phospholipase D mediates very low-density lipoprotein-induced aldosterone production, in part, via lipin-1.J Mol Endocrinol. 2023 Mar 27;70(4):e220196. doi: 10.1530/JME-22-0196. Print 2023 May 1. J Mol Endocrinol. 2023. PMID: 36779781
-
Endothelin-1 stimulates hydrolysis of phosphatidylcholine by phospholipases C and D in intact rat mesenteric arteries.J Vasc Res. 1999 Jan-Feb;36(1):35-46. doi: 10.1159/000025624. J Vasc Res. 1999. PMID: 10050072
Cited by
-
Multi-Omic Analysis of the Differences in Growth and Metabolic Mechanisms Between Chinese Domestic Cattle and Simmental Crossbred Cattle.Int J Mol Sci. 2025 Feb 12;26(4):1547. doi: 10.3390/ijms26041547. Int J Mol Sci. 2025. PMID: 40004011 Free PMC article.
-
The impact of lipid metabolism on breast cancer: a review about its role in tumorigenesis and immune escape.Cell Commun Signal. 2023 Jun 27;21(1):161. doi: 10.1186/s12964-023-01178-1. Cell Commun Signal. 2023. PMID: 37370164 Free PMC article. Review.
-
Determination of endogenous sphingolipid content in stroke rats and HT22 cells subjected to oxygen-glucose deprivation by LC‒MS/MS.Lipids Health Dis. 2023 Jan 25;22(1):13. doi: 10.1186/s12944-022-01762-3. Lipids Health Dis. 2023. PMID: 36698123 Free PMC article.
-
A novel signature incorporating genes related to lipid metabolism and immune for prognostic and functional prediction of breast cancer.Aging (Albany NY). 2024 May 20;16(10):8611-8629. doi: 10.18632/aging.205828. Epub 2024 May 20. Aging (Albany NY). 2024. PMID: 38771140 Free PMC article.
-
Role of sphingolipid metabolites in the homeostasis of steroid hormones and the maintenance of testicular functions.Front Endocrinol (Lausanne). 2023 Mar 17;14:1170023. doi: 10.3389/fendo.2023.1170023. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37008929 Free PMC article. Review.
References
-
- Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Jr., Collins K.J., Dennison Himmelfarb C., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., MacLaughlin E.J., Muntner P., Ovbiagele B., Smith S.C., Jr., Spencer C.C., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. - PubMed
-
- Hannemann A., Wallaschofski H. Prevalence of primary aldosteronism in patient's cohorts and in population-based studies--a review of the current literature. Horm. Metab. Res. 2012;44:157–162. - PubMed
-
- Käyser S.C., Dekkers T., Groenewoud H.J., van der Wilt G.J., Carel Bakx J., van der Wel M.C., Hermus A.R., Lenders J.W., Deinum J. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J. Clin. Endocrinol. Metab. 2016;101:2826–2835. - PubMed
-
- Briet M., Schiffrin E.L. The role of aldosterone in the metabolic syndrome. Curr. Hypertens. Rep. 2011;13:163–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

