Heat and cold denaturation of yeast frataxin: The effect of pressure
- PMID: 35278425
- PMCID: PMC9072581
- DOI: 10.1016/j.bpj.2022.03.010
Heat and cold denaturation of yeast frataxin: The effect of pressure
Abstract
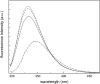
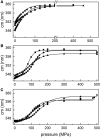
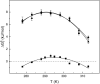
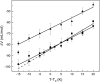
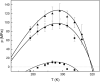
Yfh1 is a yeast protein with the peculiar characteristic to undergo, in the absence of salt, cold denaturation at temperatures above the water freezing point. This feature makes the protein particularly interesting for studies aiming at understanding the rules that determine protein fold stability. Here, we present the phase diagram of Yfh1 unfolding as a function of pressure (0.1-500 MPa) and temperature 278-313 K (5-40°C) both in the absence and in the presence of stabilizers using Trp fluorescence as a monitor. The protein showed a remarkable sensitivity to pressure: at 293 K, pressures around 10 MPa are sufficient to cause 50% of unfolding. Higher pressures were required for the unfolding of the protein in the presence of stabilizers. The phase diagram on the pressure-temperature plane together with a critical comparison between our results and those found in the literature allowed us to draw conclusions on the mechanism of the unfolding process under different environmental conditions.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Hopkins F.G. Denaturation of proteins by urea and related substances. Nature. 1930;126:328–330.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

