Gonadal white adipose tissue is important for gametogenesis in mice through maintenance of local metabolic and immune niches
- PMID: 35278432
- PMCID: PMC9052151
- DOI: 10.1016/j.jbc.2022.101818
Gonadal white adipose tissue is important for gametogenesis in mice through maintenance of local metabolic and immune niches
Abstract
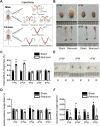
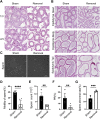
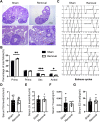
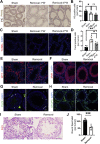
Gonadal white adipose tissue (gWAT) can regulate gametogenesis via modulation of neuroendocrine signaling. However, the effect of gWAT on the local microenvironment of the gonad was largely unknown. Herein, we ruled out that gWAT had a neuroendocrine effect on gonad function through a unilateral lipectomy strategy, in which cutting off epididymal white adipose tissue could reduce seminiferous tubule thickness and decrease sperm counts only in the adjacent testis and epididymis of the affected gonad. Consistent with the results in males, in females, ovary mass was similarly decreased by lipectomy. We determined that the defects in spermatogenesis were mainly caused by augmented apoptosis and decreased proliferation of germ cells. Transcriptome analysis suggested that lipectomy could disrupt immune privilege and activate immune responses in both the testis and ovary on the side of the lipectomy. In addition, lipidomics analysis in the testis showed that the levels of lipid metabolites such as free carnitine were elevated, whereas the levels of glycerophospholipids such as phosphatidylcholines and phosphatidylethanolamines were decreased, which indicated that the metabolic niche was also altered. Finally, we show that supplementation of phosphatidylcholine and phosphatidylethanolamine could partially rescue the observed phenotype. Collectively, our findings suggest that gWAT is important for gonad function by not only affecting whole-body homeostasis but also via maintaining local metabolic and immune niches.
Keywords: gWAT; gametogenesis; immune niche; lipectomy; metabolic niche.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Du Plessis S.S., Cabler S., McAlister D.A., Sabanegh E., Agarwal A. The effect of obesity on sperm disorders and male infertility. Nat. Rev. Urol. 2010;7:153–161. - PubMed
-
- Pasquali R., Pelusi C., Genghini S., Cacciari M., Gambineri A. Obesity and reproductive disorders in women. Hum. Reprod. Update. 2003;9:359–372. - PubMed
-
- Yen S.S. Female hypogonadotropic hypogonadism. Hypothalamic amenorrhea syndrome. Endocrinol. Metab. Clin. North Am. 1993;22:29–58. - PubMed
-
- Starkey T.A., Lee R.A. Menstruation and fertility in anorexia nervosa. Am. J. Obstet. Gynecol. 1969;105:374–379. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

