In vitro inhibition of carboxylesterase 1 by Kava (Piper methysticum) Kavalactones
- PMID: 35278473
- PMCID: PMC9244838
- DOI: 10.1016/j.cbi.2022.109883
In vitro inhibition of carboxylesterase 1 by Kava (Piper methysticum) Kavalactones
Abstract
Kava refers to the extracts from the rhizome of the plant Piper methysticum which is of particular significance to various indigenous cultures in the South Pacific region. Kavalactones are the active constituents of kava products and are associated with sedative and anxiolytic effects. Kavalactones have been evaluated in vitro for their potential to alter the activity of various CYP450 enzymes but have undergone little systematic investigation as to their potential influence on esterases. This study investigated the inhibition effects of kava and its kavalactones on carboxylesterase 1 (CES1) in an in vitro system and established associated kinetic parameters. Kava and its kavalactones were found to produce reversible inhibition of CES1 to varying degrees. Kavain, dihydrokavain, and desmethoxyyangonin displayed competitive type inhibition, while methysticin, dihydromethysticin, and yangonin displayed a mixed competitive-noncompetitive type inhibition. The inhibition constants (Ki) values for each of the kavalactones were as follows: methysticin (35.2 μM), dihydromethysticin (68.2 μM), kavain (81.6 μM), dihydrokavain (105.3 μM), yangonin (24.9 μM), and desmethoxyyangonin (25.2 μM). With consideration to the in vitro Ki for each evaluated kavalactone as well as available clinical kavalactone concentrations in blood circulation, co-administration of CES1 substrate medications and kava products at the recommended daily dose is generally free of drug interaction concerns. However, uncertainty around kavalactone exposure in humans has been noted and a clinically relevant CES1 inhibition by kavain, dihydrokavain, and dihydromethysticin is indeed possible if the kavalactone consumption is higher than 1000 mg in the context of over-the-counter usage. Further clinical studies would be required to assess the possibility of clinically significant kava drug-drug interactions with CES1 substrate medications.
Keywords: CES1; Inhibition; Interaction; Kavalactones; Piper methysticum.
Copyright © 2022 Elsevier B.V. All rights reserved.
Figures
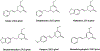



References
-
- Smith T, Majid F, Eckl V, Reynolds CM, Herbal Supplement Sales in US Increase by Record-Breaking 17.3% in 2020, HerbalGram, 2021, p. 14. https://www.herbalgram.org/resources/herbalgram/issues/131/table-of-cont....
-
- Lebot V, Merlin M, Lindstrom L, Kava: the Pacific Elixir: the Definitive Guide to its Ethnobotany, History, and Chemistry, Inner Traditions/Bear & Co, 1997.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

