Using OPM-MEG in contrasting magnetic environments
- PMID: 35278706
- PMCID: PMC9135301
- DOI: 10.1016/j.neuroimage.2022.119084
Using OPM-MEG in contrasting magnetic environments
Abstract
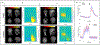
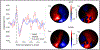
Magnetoencephalography (MEG) has been revolutionised by optically pumped magnetometers (OPMs). "OPM-MEG" offers higher sensitivity, better spatial resolution, and lower cost than conventional instrumentation based on superconducting quantum interference devices (SQUIDs). Moreover, because OPMs are small, lightweight, and portable they offer the possibility of lifespan compliance and (with control of background field) motion robustness, dramatically expanding the range of MEG applications. However, OPM-MEG remains nascent technology; it places stringent requirements on magnetic shielding, and whilst a number of viable systems exist, most are custom made and there have been no cross-site investigations showing the reliability of data. In this paper, we undertake the first cross-site OPM-MEG comparison, using near identical commercial systems scanning the same participant. The two sites are deliberately contrasting, with different magnetic environments: a "green field" campus university site with an OPM-optimised shielded room (low interference) and a city centre hospital site with a "standard" (non-optimised) MSR (higher interference). We show that despite a 20-fold difference in background field, and a 30-fold difference in low frequency interference, using dynamic field control and software-based suppression of interference we can generate comparable noise floors at both sites. In human data recorded during a visuo-motor task and a face processing paradigm, we were able to generate similar data, with source localisation showing that brain regions could be pinpointed with just ∼10 mm spatial discrepancy and temporal correlations of > 80%. Overall, our study demonstrates that, with appropriate field control, OPM-MEG systems can be sited even in city centre hospital locations. The methods presented pave the way for wider deployment of OPM-MEG.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Conflicts of interest V.S. is the founding director of QuSpin, the commercial entity selling OPM magnetometers. J.O. is an employee of QuSpin. E.B. and M.J.B. are directors of Cerca Magnetics Limited, a spin-out company whose aim is to commercialise aspects of OPM-MEG technology. E.B., M.J.B., R.B., N.H. and R.H. hold founding equity in Cerca Magnetics Limited.
Figures
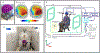



References
-
- Altarev I, Fierlinger P, Lins T, Marino MG, Nießen B, Petzoldt G, Reisner M, Stuiber S, Sturm M, Taggart Singh J, Taubenheim B, Rohrer HK, Schläpfer U, 2015. Minimizing magnetic fields for precision experiments. J. Appl. Phys 117 (23). doi:10.1063/1.4922671. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

