Lower persistence of anti-nucleocapsid compared to anti-spike antibodies up to one year after SARS-CoV-2 infection
- PMID: 35278794
- PMCID: PMC8837483
- DOI: 10.1016/j.diagmicrobio.2022.115659
Lower persistence of anti-nucleocapsid compared to anti-spike antibodies up to one year after SARS-CoV-2 infection
Abstract
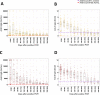
We retrospectively compared the long-term evolution of IgG anti-spike (S) and anti-nucleocapsid (N) levels (Abbott immunoassays) in 116 non-severe and 115 severe SARS-CoV-2 infected patients from 2 university hospitals up to 365 days post positive RT-PCR. IgG anti-S and anti-N antibody levels decayed exponentially up to 365 days after a peak 0 to 59 days after positive RT-PCR. Peak antibody level/cut-off ratio 0 to 59 days after positive RT-PCR was more than 70 for anti-S compared to less than 6 for anti-N (P < 0.01). Anti-S and anti-N were significantly higher in severe compared to non-severe patients up to 180 to 239 days and 300 to 365 days, respectively (P < 0.05). Despite similar half-lives, the estimated time to 50% seronegativity was more than 2 years for anti-S compared to less than 1 year for anti-N in non-severe and severe COVID-19 patients, due to the significantly higher peak antibody level/cut-off ratio for anti-S compared to anti-N.
Keywords: COVID-19; COVID-19 testing; IgG; Immunoassay; Nucleocapsid; SARS-CoV-2; Serological Testing; Spike.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interests Dr. Vermeersch reports personal fees from Roche, outside the submitted work. Dr. Lagrou reports personal fees and nonfinancial support from Pfizer, personal fees and nonfinancial support from MSD, personal fees from SMB Laboratoires, personal fees from Gilead, and personal fees from FUJIFILM Wako, outside the submitted work. The other authors state no conflicts of interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

