Real-world evaluation of rapid and laboratory-free COVID-19 triage for emergency care: external validation and pilot deployment of artificial intelligence driven screening
- PMID: 35279399
- PMCID: PMC8906813
- DOI: 10.1016/S2589-7500(21)00272-7
Real-world evaluation of rapid and laboratory-free COVID-19 triage for emergency care: external validation and pilot deployment of artificial intelligence driven screening
Abstract
Background: Uncertainty in patients' COVID-19 status contributes to treatment delays, nosocomial transmission, and operational pressures in hospitals. However, the typical turnaround time for laboratory PCR remains 12-24 h and lateral flow devices (LFDs) have limited sensitivity. Previously, we have shown that artificial intelligence-driven triage (CURIAL-1.0) can provide rapid COVID-19 screening using clinical data routinely available within 1 h of arrival to hospital. Here, we aimed to improve the time from arrival to the emergency department to the availability of a result, do external and prospective validation, and deploy a novel laboratory-free screening tool in a UK emergency department.
Methods: We optimised our previous model, removing less informative predictors to improve generalisability and speed, developing the CURIAL-Lab model with vital signs and readily available blood tests (full blood count [FBC]; urea, creatinine, and electrolytes; liver function tests; and C-reactive protein) and the CURIAL-Rapide model with vital signs and FBC alone. Models were validated externally for emergency admissions to University Hospitals Birmingham, Bedfordshire Hospitals, and Portsmouth Hospitals University National Health Service (NHS) trusts, and prospectively at Oxford University Hospitals, by comparison with PCR testing. Next, we compared model performance directly against LFDs and evaluated a combined pathway that triaged patients who had either a positive CURIAL model result or a positive LFD to a COVID-19-suspected clinical area. Lastly, we deployed CURIAL-Rapide alongside an approved point-of-care FBC analyser to provide laboratory-free COVID-19 screening at the John Radcliffe Hospital (Oxford, UK). Our primary improvement outcome was time-to-result, and our performance measures were sensitivity, specificity, positive and negative predictive values, and area under receiver operating characteristic curve (AUROC).
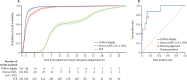
Findings: 72 223 patients met eligibility criteria across the four validating hospital groups, in a total validation period spanning Dec 1, 2019, to March 31, 2021. CURIAL-Lab and CURIAL-Rapide performed consistently across trusts (AUROC range 0·858-0·881, 95% CI 0·838-0·912, for CURIAL-Lab and 0·836-0·854, 0·814-0·889, for CURIAL-Rapide), achieving highest sensitivity at Portsmouth Hospitals (84·1%, Wilson's 95% CI 82·5-85·7, for CURIAL-Lab and 83·5%, 81·8-85·1, for CURIAL-Rapide) at specificities of 71·3% (70·9-71·8) for CURIAL-Lab and 63·6% (63·1-64·1) for CURIAL-Rapide. When combined with LFDs, model predictions improved triage sensitivity from 56·9% (51·7-62·0) for LFDs alone to 85·6% with CURIAL-Lab (81·6-88·9; AUROC 0·925) and 88·2% with CURIAL-Rapide (84·4-91·1; AUROC 0·919), thereby reducing missed COVID-19 cases by 65% with CURIAL-Lab and 72% with CURIAL-Rapide. For the prospective deployment of CURIAL-Rapide, 520 patients were enrolled for point-of-care FBC analysis between Feb 18 and May 10, 2021, of whom 436 received confirmatory PCR testing and ten (2·3%) tested positive. Median time from arrival to a CURIAL-Rapide result was 45 min (IQR 32-64), 16 min (26·3%) sooner than with LFDs (61 min, 37-99; log-rank p<0·0001), and 6 h 52 min (90·2%) sooner than with PCR (7 h 37 min, 6 h 5 min to 15 h 39 min; p<0·0001). Classification performance was high, with sensitivity of 87·5% (95% CI 52·9-97·8), specificity of 85·4% (81·3-88·7), and negative predictive value of 99·7% (98·2-99·9). CURIAL-Rapide correctly excluded infection for 31 (58·5%) of 53 patients who were triaged by a physician to a COVID-19-suspected area but went on to test negative by PCR.
Interpretation: Our findings show the generalisability, performance, and real-world operational benefits of artificial intelligence-driven screening for COVID-19 over standard-of-care in emergency departments. CURIAL-Rapide provided rapid, laboratory-free screening when used with near-patient FBC analysis, and was able to reduce the number of patients who tested negative for COVID-19 but were triaged to COVID-19-suspected areas.
Funding: The Wellcome Trust, University of Oxford Medical and Life Sciences Translational Fund.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests DWE reports personal fees from Gilead, outside the submitted work. DAC reports personal fees from Oxford University Innovation, BioBeats, and Sensyne Health; and participation on a data safety monitoring board or advisory board for Bristol Myers Squibb, outside the submitted work. All other authors declare no competing interests.
Figures



References
-
- UK National Medical Director. Chief Nursing Officer for England. Chief People Officer & National Director for Emergency and Elective Care Letter: healthcare associated COVID-19 infections—further action. 2020. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

