Patient-reported outcomes one year after positive sentinel lymph node biopsy with or without axillary lymph node dissection in the randomized SENOMAC trial
- PMID: 35279508
- PMCID: PMC8920917
- DOI: 10.1016/j.breast.2022.02.013
Patient-reported outcomes one year after positive sentinel lymph node biopsy with or without axillary lymph node dissection in the randomized SENOMAC trial
Abstract
Introduction: This report evaluates whether health related quality of life (HRQoL) and patient-reported arm morbidity one year after axillary surgery are affected by the omission of axillary lymph node dissection (ALND).
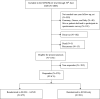
Methods: The ongoing international non-inferiority SENOMAC trial randomizes clinically node-negative breast cancer patients (T1-T3) with 1-2 sentinel lymph node (SLN) macrometastases to completion ALND or no further axillary surgery. For this analysis, the first 1181 patients enrolled in Sweden and Denmark between March 2015, and June 2019, were eligible. Data extraction from the trial database was on November 2020. This report covers the secondary outcomes of the SENOMAC trial: HRQoL and patient-reported arm morbidity. The EORTC QLQ-C30, EORTC QLQ-BR23 and Lymph-ICF questionnaires were completed in the early postoperative phase and at one-year follow-up. Adjusted one-year mean scores and mean differences between the groups are presented corrected for multiple testing.
Results: Overall, 976 questionnaires (501 in the SLN biopsy only group and 475 in the completion ALND group) were analysed, corresponding to a response rate of 82.6%. No significant group differences in overall HRQoL were identified. Participants receiving SLN biopsy only, reported significantly lower symptom scores on the EORTC subscales of pain, arm symptoms and breast symptoms. The Lymph-ICF domain scores of physical function, mental function and mobility activities were significantly in favour of the SLN biopsy only group.
Conclusion: One year after surgery, arm morbidity is significantly worse affected by ALND than by SLN biopsy only. The results underline the importance of ongoing attempts to safely de-escalate axillary surgery.
Trial registration: The trial was registered at clinicaltrials.gov prior to initiation (https://clinicaltrials.gov/ct2/show/NCT02240472).
Keywords: Arm morbidity; Axillary lymph node dissection; Breast cancer; Health-related quality of life; Patient-reported outcome measures; Sentinel lymph node biopsy.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
All listed authors declare that they have no conflict of interests.
Figures


References
-
- de Boniface J., Frisell J., Bergkvist L., Andersson Y. Swedish Breast Cancer Group and the Swedish Society of Breast Surgery. Ten-year report on axillary recurrence after negative sentinel node biopsy for breast cancer from the Swedish Multicentre Cohort Study. Br J Surg. 2017;104(3):238–247. https://doi:10.1002/bjs.10411 - DOI - PubMed
-
- Krag D.N., Anderson S.J., Julian T.B., Brown A.M., Harlow S.P., Costantino J.P., et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–933. https://doi:10.1016/s1470-2045(10)70207-2 - DOI - PMC - PubMed
-
- Veronesi U., Viale G., Paganelli G., Zurrida S., Luini A., Galimberti V., et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251(4):595–600. https://doi:10.1097/SLA.0b013e3181c0e92a - DOI - PubMed
-
- Galimberti V., Cole B.F., Viale G., Veronesi P., Vicini E., Intra M., et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2018;19(10):1385–1393. https://doi:10.1016/s1470-2045(18)30380-2 - DOI - PubMed
-
- Giuliano A.E., Ballman K.V., McCall L., Beitsch P.D., Brennan M.B., Kelemen P.R., et al. Effect of axillary dissection vs No axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial. JAMA. 2017;318(10):918–926. https://doi:10.1001/jama.2017.11470 - DOI - PMC - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

