Immune phenotype of the CD4+ T cells in the aged lymphoid organs and lacrimal glands
- PMID: 35279788
- PMCID: PMC9617003
- DOI: 10.1007/s11357-022-00529-z
Immune phenotype of the CD4+ T cells in the aged lymphoid organs and lacrimal glands
Abstract
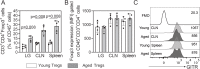
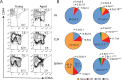
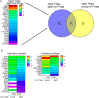
Aging is associated with a massive infiltration of T lymphocytes in the lacrimal gland. Here, we aimed to characterize the immune phenotype of aged CD4+ T cells in this tissue as compared with lymphoid organs. To perform this, we sorted regulatory T cells (Tregs, CD4+CD25+GITR+) and non-Tregs (CD4+CD25negGITRneg) in lymphoid organs from female C57BL/6J mice and subjected these cells to an immunology NanoString® panel. These results were confirmed by flow cytometry, live imaging, and tissue immunostaining in the lacrimal gland. Importantly, effector T helper 1 (Th1) genes were highly upregulated on aged Tregs, including the master regulator Tbx21. Among the non-Tregs, we also found a significant increase in the levels of EOMESmed/high, TbetnegIFN-γ+, and CD62L+CD44negCD4+ T cells with aging, which are associated with cell exhaustion, immunopathology, and the generation of tertiary lymphoid tissue. At the functional level, aged Tregs from lymphoid organs are less able to decrease proliferation and IFN-γ production of T responders at any age. More importantly, human lacrimal glands (age range 55-81 years) also showed the presence of CD4+Foxp3+ cells. Further studies are needed to propose potential molecular targets to avoid immune-mediated lacrimal gland dysfunction with aging.
Keywords: Aging; CD4+ T cells; Conventional T cells; Dry eye disease; Lacrimal gland; Lymphoid organs; Regulatory T cells; Sjögren’s syndrome; Transcriptome.
© 2022. The Author(s), under exclusive licence to American Aging Association.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

