Simultaneous Nail and Skin Clearance in Ixekizumab Head-to-Head Trials for Moderate-to-Severe Psoriasis and Psoriatic Arthritis
- PMID: 35279805
- PMCID: PMC9021345
- DOI: 10.1007/s13555-022-00704-2
Simultaneous Nail and Skin Clearance in Ixekizumab Head-to-Head Trials for Moderate-to-Severe Psoriasis and Psoriatic Arthritis
Abstract
Introduction: The lifetime incidence of nail psoriasis in patients with psoriasis is 80-90%, with 23-27% of patients having nail psoriasis at any given time. Nail psoriasis is even more prevalent in patients with comorbid psoriatic arthritis. Complete psoriasis clearance, an achievable therapeutic goal, should ideally include the resolution of nail psoriasis. Here, we assessed simultaneous skin and nail clearance in patients with psoriasis across five head-to-head trials comparing ixekizumab with other biologics.
Methods: Data were assessed in patients with moderate-to-severe psoriasis (with or without psoriatic arthritis) with nail psoriasis at baseline from the IXORA-R, IXORA-S, UNCOVER-2, UNCOVER-3, and SPIRIT-H2H trials. Ixekizumab patients received IXEQ2W to week 12 and IXEQ4W beyond week 12. PASI 100 depicted complete skin clearance, and PGA-F 0 (IXORA-R) or NAPSI 0 (all other trials) depicted complete nail clearance. Treatment comparisons were evaluated using the Cochran-Mantel-Haenszel test. Non-responder imputation was used for missing data.
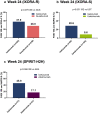
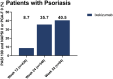
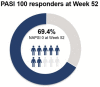
Results: Ixekizumab achieved significantly greater simultaneous skin and nail complete clearance than etanercept (UNCOVER-2: p < 0.001 and UNCOVER-3: p < 0.001) at week 12, demonstrating an efficacious and rapid response. Across all five head-to-head trials, ixekizumab achieved a high rate of simultaneous skin and nail clearance (range: 28.6-45.9% of patients) by week 24 that was maintained up to week 52 (range: 40.5-51.4% of patients). Ixekizumab achieved numerically greater simultaneous complete clearance than guselkumab at week 24 (p = 0.079), but statistically significant greater simultaneous clearance compared to ustekinumab (p < 0.001) and adalimumab (p = 0.006) at week 24 and week 52 (p < 0.001 and p = 0.007, respectively).
Conclusion: In five head-to-head trials, ixekizumab-treated patients had higher rates of simultaneous complete skin and nail clearance compared to etanercept, guselkumab, ustekinumab, and adalimumab, thereby reinforcing ixekizumab's ability to achieve high levels of efficacy in multiple domains of psoriatic disease.
Trial registration: NCT01474512, NCT01597245, NCT01646177, NCT03573323, NCT02561806, and NCT03151551.
Keywords: Adalimumab; Etanercept; Guselkumab; IL 17A inhibitor; Ixekizumab; Moderate-to-severe psoriasis; Psoriatic arthritis; Ustekinumab.
© 2022. The Author(s).
Figures


References
Associated data
LinkOut - more resources
Full Text Sources
Medical

