Synergistic use of glycomics and single-molecule molecular inversion probes for identification of congenital disorders of glycosylation type-1
- PMID: 35279850
- PMCID: PMC9545396
- DOI: 10.1002/jimd.12496
Synergistic use of glycomics and single-molecule molecular inversion probes for identification of congenital disorders of glycosylation type-1
Abstract
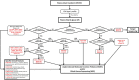
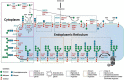
Congenital disorders of glycosylation type 1 (CDG-I) comprise a group of 27 genetic defects with heterogeneous multisystem phenotype, mostly presenting with nonspecific neurological symptoms. The biochemical hallmark of CDG-I is a partial absence of complete N-glycans on transferrin. However, recent findings of a diagnostic N-tetrasaccharide for ALG1-CDG and increased high-mannose N-glycans for a few other CDG suggested the potential of glycan structural analysis for CDG-I gene discovery. We analyzed the relative abundance of total plasma N-glycans by high resolution quadrupole time-of-flight mass spectrometry in a large cohort of 111 CDG-I patients with known (n = 75) or unsolved (n = 36) genetic cause. We designed single-molecule molecular inversion probes (smMIPs) for sequencing of CDG-I candidate genes on the basis of specific N-glycan signatures. Glycomics profiling in patients with known defects revealed novel features such as the N-tetrasaccharide in ALG2-CDG patients and a novel fucosylated N-pentasaccharide as specific glycomarker for ALG1-CDG. Moreover, group-specific high-mannose N-glycan signatures were found in ALG3-, ALG9-, ALG11-, ALG12-, RFT1-, SRD5A3-, DOLK-, DPM1-, DPM3-, MPDU1-, ALG13-CDG, and hereditary fructose intolerance. Further differential analysis revealed high-mannose profiles, characteristic for ALG12- and ALG9-CDG. Prediction of candidate genes by glycomics profiling in 36 patients with thus far unsolved CDG-I and subsequent smMIPs sequencing led to a yield of solved cases of 78% (28/36). Combined plasma glycomics profiling and targeted smMIPs sequencing of candidate genes is a powerful approach to identify causative mutations in CDG-I patient cohorts.
Keywords: CDG type 1 (CDG-I); congenital disorders of glycosylation (CDG); diagnostics by mass spectrometry; glycomics; multi-omics; smMIPs.
© 2022 The Authors. Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
All the authors declare that they have no conflict of interest and no financial relationships relevant to this article to disclose
Figures


References
-
- van Scherpenzeel M, Steenbergen G, Morava E, Wevers RA, Lefeber DJ. High‐resolution mass spectrometry glycoprofiling of intact transferrin for diagnosis and subtype identification in the congenital disorders of glycosylation. Transl Res. 2015;166(6):639‐649.e1. doi:10.1016/j.trsl.2015.07.005 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

