Health status improvement with ferric carboxymaltose in heart failure with reduced ejection fraction and iron deficiency
- PMID: 35279929
- PMCID: PMC9313582
- DOI: 10.1002/ejhf.2478
Health status improvement with ferric carboxymaltose in heart failure with reduced ejection fraction and iron deficiency
Erratum in
-
Corrigendum to: 'A comprehensive characterization of acute heart failure with preserved versus mildly reduced versus reduced ejection fraction - insights from the ESC-HFA EORP Heart Failure Long-Term Registry' and articles listed below.Eur J Heart Fail. 2023 Mar;25(3):443. doi: 10.1002/ejhf.2789. Epub 2023 Feb 17. Eur J Heart Fail. 2023. PMID: 36799232 Free PMC article. No abstract available.
Abstract
Aim: Intravenous ferric carboxymaltose (FCM) has been shown to improve overall quality of life in iron-deficient heart failure with reduced ejection fraction (HFrEF) patients at a trial population level. This FAIR-HF and CONFIRM-HF pooled analysis explored the likelihood of individual improvement or deterioration in Kansas City Cardiomyopathy Questionnaire (KCCQ) domains with FCM versus placebo and evaluated the stability of this response over time.
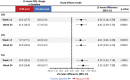
Methods and results: Changes versus baseline in KCCQ overall summary score (OSS), clinical summary score (CSS) and total symptom score (TSS) were assessed at weeks 12 and 24 in FCM and placebo groups. Mean between-group differences were estimated and individual responder analyses and analyses of response stability were performed. Overall, 760 (FCM, n = 454) patients were studied. At week 12, the mean improvement in KCCQ OSS was 10.6 points with FCM versus 4.8 points with placebo (least-square mean difference [95% confidence interval, CI] 4.36 [2.14; 6.59] points). A higher proportion of patients on FCM versus placebo experienced a KCCQ OSS improvement of ≥5 (58.3% vs. 43.5%; odds ratio [95% CI] 1.81 [1.30; 2.51]), ≥10 (42.4% vs. 29.3%; 1.73 [1.23; 2.43]) or ≥15 (32.1% vs. 22.6%; 1.46 [1.02; 2.11]) points. Differences were similar at week 24 and for CSS and TSS domains. Of FCM patients with a ≥5-, ≥10- or ≥15-point improvement in KCCQ OSS at week 12, >75% sustained this improvement at week 24.
Conclusion: Treatment of iron-deficient HFrEF patients with intravenous FCM conveyed clinically relevant improvements in health status at an individual-patient level; benefits were sustained over time in most patients.
Keywords: Ferric carboxymaltose; Health status; Heart failure with reduced ejection fraction; Iron deficiency; Kansas City Cardiomyopathy Questionnaire; Minimal clinically important difference; Quality of life.
© 2022 The Authors. European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures


References
-
- Rocha BML, Cunha GJL, Menezes Falcão LF. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol. 2018;71:782–93. - PubMed
-
- Enjuanes C, Klip IT, Bruguera J, Cladellas M, Ponikowski P, Banasiak W, et al. Iron deficiency and health‐related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol. 2014;174:268–75. - PubMed
-
- Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. 2011;17:899–906. - PubMed
-
- Marchi G, Busti F, Vianello A, Girelli D. Anemia and iron deficiency in heart failure: extending evidences from chronic to acute setting. Intern Emerg Med. 2021;16:167–70. - PubMed
-
- Martens P, Nijst P, Verbrugge FH, Smeets K, Dupont M, Mullens W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid‐range and preserved ejection fraction. Acta Cardiol. 2018;73:115–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

