N-Acetylcysteine Mitigates Social Dysfunction in a Rat Model of Autism Normalizing Glutathione Imbalance and the Altered Expression of Genes Related to Synaptic Function in Specific Brain Areas
- PMID: 35280167
- PMCID: PMC8916240
- DOI: 10.3389/fpsyt.2022.851679
N-Acetylcysteine Mitigates Social Dysfunction in a Rat Model of Autism Normalizing Glutathione Imbalance and the Altered Expression of Genes Related to Synaptic Function in Specific Brain Areas
Abstract
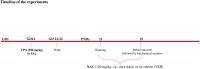
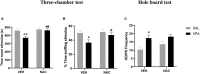
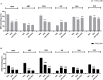
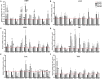
Prenatal exposure to valproic acid (VPA) is a risk factor for autism spectrum disorder (ASD) in humans and it induces autistic-like behaviors in rodents. Imbalances between GABAergic and glutamatergic neurotransmission and increased oxidative stress together with altered glutathione (GSH) metabolism have been hypothesized to play a role in both VPA-induced embriotoxicity and in human ASD. N-acetylcysteine (NAC) is an antioxidant precursor of glutathione and a modulator of glutamatergic neurotransmission that has been tested in ASD, although the clinical studies currently available provided controversial results. Here, we explored the effects of repeated NAC (150 mg/kg) administration on core autistic-like features and altered brain GSH metabolism in the VPA (500 mg/kg) rat model of ASD. Furthermore, we measured the mRNA expression of genes encoding for scaffolding and transcription regulation proteins, as well as the subunits of NMDA and AMPA receptors and metabotropic glutamate receptors mGLUR1 and mGLUR5 in brain areas that are relevant to ASD. NAC administration ameliorated the social deficit displayed by VPA-exposed rats in the three-chamber test, but not their stereotypic behavior in the hole board test. Furthermore, NAC normalized the altered GSH levels displayed by these animals in the hippocampus and nucleus accumbens, and it partially rescued the altered expression of post-synaptic terminal network genes found in VPA-exposed rats, such as NR2a, MGLUR5, GLUR1, and GLUR2 in nucleus accumbens, and CAMK2, NR1, and GLUR2 in cerebellum. These data indicate that NAC treatment selectively mitigates the social dysfunction displayed by VPA-exposed rats normalizing GSH imbalance and reestablishing the expression of genes related to synaptic function in a brain region-specific manner. Taken together, these data contribute to clarify the behavioral impact of NAC in ASD and the molecular mechanisms that underlie its effects.
Keywords: N-acetylcysteine; autism; glutathione; rats; valproic acid.
Copyright © 2022 Schiavi, La Rosa, Petrillo, Carbone, D'Amico, Piemonte and Trezza.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

