Blinding and Its Quality in Clinical Trials Conducted on Patients with Breast Cancer: A Systematic Review
- PMID: 35280192
- PMCID: PMC8865247
- DOI: 10.4103/ijnmr.IJNMR_49_20
Blinding and Its Quality in Clinical Trials Conducted on Patients with Breast Cancer: A Systematic Review
Abstract
Background: Blinding is one of the critical criteria of clinical trials that prevents probable bias. Judgment regarding results of an intervention significantly depends on the quality of such studies, one of which is blinding. This study aimed to investigate blinding and its quality in clinical trials in patients with breast cancer.
Materials and methods: A systematic review was conducted on the online databases of PubMed, ScienceDirect and ProQuest using keywords, MeSH terms and grey literature. Articles were screened by predefined inclusion and exclusion criteria. They were evaluated based on the checklists introduced by Cochrane database.
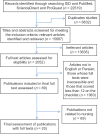
Results: From 22519 articles obtained at the initial stage, 20 articles remained after screening for the inclusion and exclusion criteria. Fifteen articles had used single, five: double and none had used triple or quadruple blinding. Seventeen studies had described the details of blinding. Of the 15 single blind articles, the blinded subjects were patients in five, patients and research assistants in three, research assistants in five studies, and two had not given any details.
Conclusions: The majority of researchers had used the single blind method, though using double, triple or quadruple blinding increases the trustworthiness of results and increases the quality of clinical trials. The details of blinding should be explained to other researchers and for a better understanding of the method if it is to be repeated. Thereafter, nurses can apply new interventions and earn their patients' trust and help those with breast cancer by relieving them of their disease symptoms and its treatment complications.
Keywords: Breast neoplasms; clinical trial; double-blind method.
Copyright: © 2022 Iranian Journal of Nursing and Midwifery Research.
Conflict of interest statement
Nothing to declare.
Figures
References
-
- World Health Organization. Noncommunicable Disease, countries profile. [Last access on 2021 Aug 24]. Available from: https://www.who.int/nmh/publications/ncd-profiles-2018/en/
-
- Matourypour P. Cancer from Nursing Perspective. Tehran: Heidari Publication; 2017.
-
- Smeltzer SC, Bare BG, Hinke JL, Cheere KH, Kluwer W. Brunner and Sudarth's Textbook of Medical-Surgical Nursing. 16th ed. Philadelphia: Lippincot Williams and Wilkins; 2018.
-
- Matourypour P. Clinical Guideline for Nursing Students and Nurses in Patients with Breast Cancer. Tehran: Heidari Publication; 2016.
Publication types
LinkOut - more resources
Full Text Sources