Blunted Post-COVID-19 Humoral Immunity in Patients With CNS Demyelinating Disorders on Anti-CD20 Treatments
- PMID: 35280260
- PMCID: PMC8905651
- DOI: 10.3389/fneur.2022.843081
Blunted Post-COVID-19 Humoral Immunity in Patients With CNS Demyelinating Disorders on Anti-CD20 Treatments
Abstract
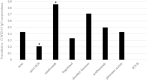
With unclear characteristics of post-infection and post-vaccination immunity, the multiple sclerosis community lacks evidence to guide patients on their continued coronavirus disease 2019 (COVID-19) infection risk. As disease modifying treatments all modulate the immune system, we expect their use to alter acquired immunity to COVID-19, but the specific impact of individual treatments is unclear. To address this, we analyzed the patient and COVID-19 specific characteristics associated with post-infection humoral immunity in 58 patients with central nervous system (CNS) demyelinating disorders in the Boston metropolitan area. Univariate analysis of variance was performed using Mann Whitney U test for continuous variables, and Chi Square or Fisher Exact test for nominal variables. Univariate and stepwise multivariate nominal logistic regression identified clinical characteristics associated with COVID-19 specific nucleocapsid IgG antibody formation post-infection. Our cohort demonstrated a 42% post-infection seropositive rate with a significantly higher rate observed with shorter duration between infection and antibody collection and use of natalizumab over no/other treatment. Use of anti-CD20 treatments compared to no/other treatment was associated with a significantly lower rate of seropositivity. However, only shorter duration between infection and antibody collection as well as use of no/other treatment compared to anti-CD20 treatment were found to be independently associated with increased likelihood of post-infection seropositivity. Additionally, we demonstrate durability of antibody response up to 9 months in a small subset of patients. Thus, our data supports that patients with CNS demyelinating disorders regardless of DMT are able to form a measurable antibody response after COVID-19 infection, and that patients on anti-CD20 treatments form less robust immunity after COVID-19 infection.
Keywords: COVID-19 antibody; anti-CD20 monoclonal antibodies; disease modifying therapy; multiple sclerosis; natalizumab.
Copyright © 2022 Money, Baber, Saart, Samaan and Sloane.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

