Intermediate-Intensity Autologous Hematopoietic Stem Cell Transplantation Reduces Serum Neurofilament Light Chains and Brain Atrophy in Aggressive Multiple Sclerosis
- PMID: 35280289
- PMCID: PMC8907141
- DOI: 10.3389/fneur.2022.820256
Intermediate-Intensity Autologous Hematopoietic Stem Cell Transplantation Reduces Serum Neurofilament Light Chains and Brain Atrophy in Aggressive Multiple Sclerosis
Abstract
Background: Autologous haematopoietic stem cell transplantation (AHSCT) is highly effective in reducing new inflammatory activity in aggressive multiple sclerosis (MS). A remarkable decrease of serum neurofilament light chains (sNfL) concentration, a marker of axonal damage, was reported in MS following high-intensity regimen AHSCT, but hints for potential neurotoxicity had emerged. sNfL and brain atrophy were therefore analysed in a cohort of patients with aggressive MS treated with intermediate-intensity AHSCT, exploring whether sNfL might be a reliable marker of disability progression independent from new inflammation (i.e. relapses and/or new/gadolinium-enhancing MRI focal lesions).
Methods: sNfL concentrations were measured using SIMOA methodology in peripheral blood from relapsing-remitting (RR-) or secondary-progressive (SP-) MS patients undergoing AHSCT (MS AHSCT), collected before transplant and at months 6 and 24 following the procedure. sNfL measured at a single timepoint in SP-MS patients not treated with AHSCT without recent inflammatory activity (SP-MS CTRL) and healthy subjects (HD) were used as controls. The rate of brain volume loss (AR-BVL) was also evaluated by MRI in MS AHSCT cases.
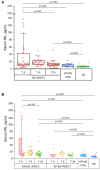
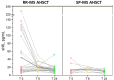
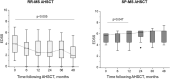
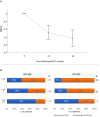
Results: Thirty-eight MS AHSCT (28 RR-MS; 10 SP-MS), 22 SP-MS CTRL and 19 HD were included. Baseline median sNfL concentrations were remarkably higher in the MS AHSCT than in the SP-MS CTRL and HD groups (p = 0.005 and <0.0001, respectively), and levels correlated with recent inflammatory activity. After a marginal (not significant) median increase observed at month 6, at month 24 following AHSCT sNfL concentrations decreased compared to baseline by median 42.8 pg/mL (range 2.4-217.3; p = 0.039), reducing by at least 50% in 13 cases, and did not differ from SP-MS CTRL (p = 0.110) but were still higher than in HD (p < 0.0001). Post-AHSCT AR-BVL normalised in 55% of RR-MS and in 30% of SP-MS. The effectiveness and safety of AHSCT were aligned with the literature.
Conclusion: sNfL concentrations correlated with recent inflammatory activity and were massively and persistently reduced by intermediate-intensity AHSCT. Association with response to treatment assessed by clinical or MRI outcomes was not observed, suggesting a good sensitivity of sNfL for recent inflammatory activity but low sensitivity in detecting ongoing axonal damage independent from new focal inflammation.
Keywords: PIRA; biomarker; brain atrophy; hematopoietic (stem) cell transplantation (HSCT); multiple sclerosis; neurofilament light (NfL); progression independent of relapse activity.
Copyright © 2022 Mariottini, Marchi, Innocenti, Di Cristinzi, Pasca, Filippini, Barilaro, Mechi, Fani, Mazzanti, Biagioli, Materozzi, Saccardi, Massacesi and Repice.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Cohen JA, Baldassari LE, Atkins HL, Bowen JD, Bredeson C, Carpenter PA, et al. Autologous hematopoietic cell transplantation for treatment-refractory relapsing multiple sclerosis: position statement from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. (2019) 25:845–54. 10.1016/j.bbmt.2019.02.014 - DOI - PubMed
-
- Sharrack B, Saccardi R, Alexander T, Badoglio M, Burman J, Farge D, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant. (2019). 10.1038/s41409-019-0684-0 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

