Development of a minimally invasive pulmonary porcine embolism model via endobronchial ultrasound
- PMID: 35280485
- PMCID: PMC8902127
- DOI: 10.21037/jtd-21-1242
Development of a minimally invasive pulmonary porcine embolism model via endobronchial ultrasound
Abstract
Background: Current massive pulmonary embolism (PE) animal models use central venous access to deliver blood clots, which have features of random clot distribution and potentially fatal hemodynamic compromise. A clinically relevant preclinical model for generating pulmonary emboli in a more controlled fashion would be of value for a variety of research studies, including initial evaluation of novel therapeutic approaches. Endobronchial ultrasound-guided transbronchial needle injection (EBUS-TBNI) is a newly established approach for peri-tracheal/bronchial targets. The purpose of the present work was to establish a minimally invasive PE model in swine via a transbronchial approach.
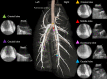
Methods: In anesthetized Yorkshire pigs, a 21-G EBUS-guided transbronchial needle aspiration (EBUS-TBNA) needle was introduced into the pulmonary artery under EBUS guidance. Autologous blood clots were administered into the right and left lower pulmonary arteries sequentially (PE1 and PE2, respectively). Hemodynamic and biochemical responses were evaluated.
Results: Ten pigs were evaluated; all 20 blood clots (6.3±1.9 mL) were successfully injected. After injection, mean pulmonary artery pressure (mPAP; mmHg) increased (baseline: 16.6±5.6 vs. PE1: 24.5±7.6, P<0.0001 vs. PE2: 26.9±6.7, P<0.0001), and a positive correlation was observed between clot volume and change in mPAP (PE1: r=0.69, P=0.025; PE1 + PE2: r=0.60, P=0.063). Mean arterial pressure (MAP; mmHg) (baseline: 57.5±5.1 vs. PE1: 59.0±9.1, P=0.918 vs. PE2: 60.9±9.6, P=0.664) remained stable. No complications were observed.
Conclusions: EBUS allows minimally invasive, precise, and reliable generation of pulmonary emboli in pigs. This model may serve as an important tool for new PE-related diagnostic and therapeutic research.
Keywords: Pulmonary embolism (PE); endobronchial ultrasound (EBUS); swine.
2022 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1242/coif). KY reports that this work was supported by a research grant from Olympus Corporation, and KY is a consultant for Olympus. The other authors have no conflicts of interest to declare.
Figures




References
LinkOut - more resources
Full Text Sources
