Increased serum free IgE levels in patients with chronic spontaneous urticaria (CSU)
- PMID: 35280503
- PMCID: PMC8883342
- DOI: 10.1016/j.waojou.2022.100629
Increased serum free IgE levels in patients with chronic spontaneous urticaria (CSU)
Abstract
Background: IgE bound on the surface of mast cells contributes to the pathogenesis of chronic spontaneous urticaria (CSU). Atopy is a predisposing factor for CSU, where omalizumab is a widely used monoclonal antibody to control urticaria symptoms via capturing serum free IgE. However, the role of serum free IgE is not clarified in CSU. The present study evaluated the clinical relevance of serum free IgE in patients with CSU.
Methods: Eighty-eight patients with CSU and 76 healthy controls (HCs) were enrolled in this study. Serum total and Dermatophagoides pteronyssinus (Der p)-specific IgE levels were measured by ImmunoCAPs. The serum free IgE levels were measured by ELISA using a novel IgETRAP, and their associations with clinical parameters, including urticaria activity score (UAS), were evaluated. Changes in serum free and total IgE levels after omalizumab treatment were observed in 23 CSU patients in comparison between responders (≥50% reduction in UAS) and non-responders (<50% reduction).
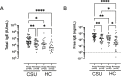
Results: Significantly higher serum free/total IgE levels were noted in CSU patients than in HCs with a positive correlation between the 2 values (rho = 0.87, P < 0.001). Among CSU patients, atopics had significantly higher serum free IgE levels than non-atopics, while no associations were noted with UAS, urticaria duration, or the results of serum ANA or autologous serum skin tests. In addition, there were no significant changes in serum free IgE levels during 12 months of omalizumab treatment. No significant differences were noted in serum free/total IgE levels or clinical parameters between responders and non-responders, while responders have higher serum Der p-specific IgE level and its ratio to serum free/total IgE level than non-responders (P < 0.05, respectively).
Conclusions: These findings suggest that increased serum free IgE may be involved in the development of CSU by activating mast cells, especially in atopics. High Der p-specific IgE level and its ratio to serum free IgE level may be a potential biomarker for predicting favorable responses to omalizumab in CSU.
Keywords: ANA, Antinuclear antibody; ASST, Autologous serum skin test; Anti-IgE antibody; Atopy; Autoimmunity; CSU, Chronic spontaneous urticaria; Chronic urticaria; ELISA, Enzyme-linked immunosorbent assay; House dust mite; IgE; Treatment; UAS, Urticaria activity score.
© 2022 The Author(s).
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures

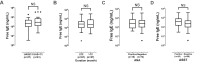

Similar articles
-
Detection of serum IgG autoantibodies to FcεRIα by ELISA in patients with chronic spontaneous urticaria.PLoS One. 2022 Aug 19;17(8):e0273415. doi: 10.1371/journal.pone.0273415. eCollection 2022. PLoS One. 2022. PMID: 35984815 Free PMC article.
-
Omalizumab treatment and outcomes in Chinese patients with chronic spontaneous urticaria, chronic inducible urticaria, or both.World Allergy Organ J. 2021 Jan 5;14(1):100501. doi: 10.1016/j.waojou.2020.100501. eCollection 2021 Jan. World Allergy Organ J. 2021. PMID: 33510832 Free PMC article.
-
Atopy and Response to Omalizumab Treatment in Chronic Spontaneous Urticaria.Int Arch Allergy Immunol. 2024;185(3):260-266. doi: 10.1159/000535414. Epub 2023 Dec 19. Int Arch Allergy Immunol. 2024. PMID: 38113870
-
Biomarkers of Autoimmune Chronic Spontaneous Urticaria.Curr Allergy Asthma Rep. 2023 Dec;23(12):655-664. doi: 10.1007/s11882-023-01117-7. Epub 2023 Dec 8. Curr Allergy Asthma Rep. 2023. PMID: 38064133 Review.
-
Association Between Serum Total IgE Levels and Clinical Response to Omalizumab for Chronic Spontaneous Urticaria: A Systematic Review and Meta-Analysis.J Allergy Clin Immunol Pract. 2023 Aug;11(8):2382-2389.e3. doi: 10.1016/j.jaip.2023.05.033. Epub 2023 May 30. J Allergy Clin Immunol Pract. 2023. PMID: 37263348
Cited by
-
Prognostic value of serum total IgE and FeNO levels in children with atopic constitution bronchiolitis.Sci Rep. 2024 Sep 10;14(1):21160. doi: 10.1038/s41598-024-72236-y. Sci Rep. 2024. PMID: 39256587 Free PMC article.
-
Atopy in chronic urticaria: an important yet overlooked issue.Front Immunol. 2024 Feb 6;15:1279976. doi: 10.3389/fimmu.2024.1279976. eCollection 2024. Front Immunol. 2024. PMID: 38380314 Free PMC article. Review.
-
Therapeutic Efficacy of YH35324 on FcεRIα-Mediated Mast Cell/Basophil Activation.Allergy Asthma Immunol Res. 2025 Mar;17(2):181-195. doi: 10.4168/aair.2025.17.2.181. Allergy Asthma Immunol Res. 2025. PMID: 40204504 Free PMC article.
-
Omalizumab Treated Urticaria Patients Display T Cell and Thrombocyte-Associated Gene Regulation.Immun Inflamm Dis. 2025 Feb;13(2):e70132. doi: 10.1002/iid3.70132. Immun Inflamm Dis. 2025. PMID: 39935189 Free PMC article.
-
The Role of Crosstalk of Immune Cells in Pathogenesis of Chronic Spontaneous Urticaria.Front Immunol. 2022 May 31;13:879754. doi: 10.3389/fimmu.2022.879754. eCollection 2022. Front Immunol. 2022. PMID: 35711438 Free PMC article. Review.
References
-
- Zuberbier T., Aberer W., Asero R., et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393–1414. - PubMed
LinkOut - more resources
Full Text Sources

