Therapeutic potential of mangiferin against kidney disorders and its mechanism of action: A review
- PMID: 35280538
- PMCID: PMC8913403
- DOI: 10.1016/j.sjbs.2021.11.016
Therapeutic potential of mangiferin against kidney disorders and its mechanism of action: A review
Abstract
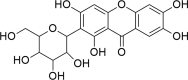
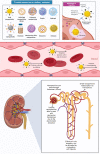
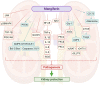
There is a swing in research developments concerning the utilization of natural products as effective pharmacotherapeutic agents due to their comparatively lower toxicities than synthetic compounds. Among natural products, mangiferin is a natural C-glucosyl xanthonoid polyphenol with remarkable pharmacological activities. Emerging evidence indicates the therapeutic benefits of mangiferin against various kidney disorders, including renal injury, diabetic nephropathy, renal fibrosis, hyperuricemic nephropathy, and lupus nephritis, in experimental animal models. The mangiferin induced antioxidant response resulting in vital functions, such as protection against renal inflammation, inhibits renal cell apoptosis, activates autophagy, causes immunomodulation, regulates renal urate transporters and modulates cell signalling pathways. The purpose of this review provide a brief overview of the in vitro/in vivo reno-protective effect of mangiferin and the underlying mechanism(s) in protecting against kidney disorders. Understanding the pharmacological actions of mangiferin is prominence due to its excellent therapeutic potential in managing kidney disorders. Thus, in addition to this review, in-silico molecular docking is performed against nuclear factor kappa B (NF-κB) and soluble epoxide hydrolase (sEH) to study the mechanism of action of mangiferin. It is believed that mangiferin is a safe reno-protective molecule. The observed positive effects are attributed to the inhibition of inflammation caused by NF-κB and sEH upregulation and oxidative stress activation. Studies on the efficacy and safety of mangiferin in clinical trials are further warranted to confirm its medicinal potential as therapeutic agent for kidney disorders in humans.
Keywords: Drug delivery; In-silico; Kidney disorders; Mangifera indica; Mangiferin; Reno-protective.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Adeola H.A., Bano A., Vats R., Vashishtha A., Verma D., Kaushik D., Mittal V., Rahman M.H., Najda A., Albadrani G.M., Sayed A.A., Farouk S.M., Hassanein E.H.M., Akhtar M.F., Saleem A., Abdel-Daim M.M., Bhardwaj R. Bioactive compounds and their libraries: An insight into prospective phytotherapeutics approach for oral mucocutaneous cancers. Biomed. Pharmacother. 2021;141:111809. - PubMed
-
- Amilkanthawar V., Daswadkar S., patil V., Joshi L. Review on molecular docking: Novel drug discovery and drug designing tool. Int. J. Eng. Res. 2020;11(4):647–665.
-
- Bastos A., Onuchic L. Molecular and cellular pathogenesis of autosomal dominant polycystic kidney disease. Braz. J. Med. Biol. Res. 2011;44:606–617. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

