Acute and sub-acute oral toxicity Lagerstroemia speciosa in Sprague-Dawley rats
- PMID: 35280577
- PMCID: PMC8913382
- DOI: 10.1016/j.sjbs.2021.11.005
Acute and sub-acute oral toxicity Lagerstroemia speciosa in Sprague-Dawley rats
Abstract
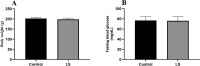
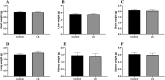
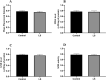
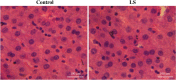
Through the boost of the natural medicinal market, individuals began to use a variety of organic materials in the marketed herbal preparation. Lagerstroemia speciosa (LS) leaves are known as banaba. People have been using a decoction of LS leaves as antidiabetic. The study aimed to investigate the acute and sub-acute oral toxicity of LS in Sprague-Dawley rats. The acute toxicity was determined by a single oral dose of LS (2000 mg/kg). Therein animal behaviour and mortality rate were observed for 14 days. The LS (200 mg/kg) was given for 28 days daily in the sub-acute study. The body weight, organ weight, food, water intake, biochemical, haematological parameters, and histopathology were studied. The findings of this study showed no mortality or morbidity was found in acute and sub-acute toxicity studies in rats. Additionally, no significant variations were found in the respective weight of organ, haematological and biochemical parameters of treated groups with reference to the control group. Moreover, no visible histological changes were detected in the liver of treated groups with reference to the control. In conclusion, the oral administration of LS did not fabricate any major toxic effect in rats. No toxic consequences were reported during acute and sub-acute toxicity investigations. Overall, LS is a safe, natural bio-actives as studied. Further investigations of cytotoxicity and genotoxicity of the above drug(s) or their combinations may be executed for appreciative safety.
Keywords: ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; Biochemical investigations; FBG, Fasting blood glucose; GSH, Reduced glutathione; Haematology; Histopathology; LS, Lagerstroemia speciosa; MDA, Malondialdehyde; ROS, Reactive oxygen species; SOD, Superoxide dismutase; Toxicity.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Abid R., Mahmood R., Rajesh K.P., Kumara Swamy B.E. Potential in vitro antioxidant and protective effect of Cassia fistula Linn. Fruit extracts against induced oxidative damage in human erythrocytes. Int. J. Pharm. Pharm. Sci. 2014
-
- Al-Mamary, M., Al-Habori, M., Al-Aghbari, A.M., Baker, M.M., 2002. Investigation into the toxicological effects of Catha edulis leaves: A short term study in animals. Phyther. Res. https://doi.org/10.1002/ptr.835. - PubMed
-
- Aneesh, T.P., Hisham, M., Sekhar, S., Madhu, M., Deepa, T. V, 2009. International market scenario of traditional Indian herbal drugs–India declining…. Int. J. Green Pharm. 3.
LinkOut - more resources
Full Text Sources

