ELIGANT: a Phase 4, interventional, safety study of leuprorelin acetate (ELIGARD®) in Asian men with prostate cancer
- PMID: 35280654
- PMCID: PMC8899139
- DOI: 10.21037/tau-21-723
ELIGANT: a Phase 4, interventional, safety study of leuprorelin acetate (ELIGARD®) in Asian men with prostate cancer
Abstract
Background: The incidence and mortality rate of men with prostate cancer have been increasing in Asia. ELIGARD® is a formulation of leuprorelin acetate whose safety and efficacy have been well-established in Western regions. However, limited safety data are available for Asian populations.
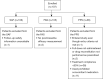
Methods: ELIGANT (ELIGard AsiaN sTudy) was a Phase 4, multicenter, prospective, single-arm, interventional study. Men with locally advanced or metastatic prostate cancer without concomitant chemotherapy, or another androgen receptor pathway inhibitor, were enrolled across Asia to receive ELIGARD® (22.5 mg subcutaneous depot injection) every 3 months for 15 months, with a follow-up visit at 18 months. The primary objective was to establish the safety of ELIGARD® in Asian men with hormone-dependent prostate cancer. The secondary objectives were to assess efficacy, via prostate-specific antigen (PSA) progression and testosterone levels, and health-related quality of life (HRQoL).
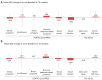
Results: In total, 106 patients were included in the safety analysis set (SAF). The most common treatment-emergent adverse events (TEAEs) included PSA increase, cough, back pain, hot flush, anemia, and upper respiratory tract infection. TEAEs considered related to ELIGARD® were reported in 13.2% of patients (n=14), two of which were serious. In the full analysis set (FAS) (n=105), 81.2% (n=56) and 68.5% (n=61) of patients achieved a PSA reduction of ≥90% from baseline at 12 and 18 months, respectively. At 18 months, the numbers of patients with testosterone levels <20, 20-50, and >50 ng/dL were 65 (61.9%), 17 (16.2%), and two (1.9%), respectively; 20% had missing testosterone measurements. HRQoL remained stable throughout the study with minimal change from baseline at study completion.
Conclusions: In conclusion, the safety profile of ELIGARD® (22.5 mg) in Asian men with hormone-dependent prostate cancer is comparable to previous studies in Western regions.
Trial registration: Clinical trial registration number NCT03035032.
Keywords: Depot formulation; leuprolide acetate; prostate cancer; prostate-specific antigen (PSA).
2022 Translational Andrology and Urology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-21-723/coif). RM was a study investigator for Astellas. RU has received a grant from Astellas during the study and personal fees for a sponsored speaker’s bureau from AstraZeneca and Takeda. JT has received grants from Baxter, Bristol-Myers Squibb, Ferring, Janssen, and Merck Sharp and Dohme and received advisory board fees from Astellas, Ferring, and Janssen during the study. JK and RP were full-time employees of Astellas during the study. EC has received a research grant and manuscript writing support from Astellas during the study, received a research grant from Janssen, received speaker honoraria from Amgen, Astellas, AstraZeneca, Bayer, Beckman Coulter Singapore Pte., Ltd., Ferring, Ipsen, and Janssen, and received advisory board fees from Amgen, Astellas, AstraZeneca, Bayer, Ferring, and Janssen. The other authors have no conflicts of interest to declare.
Figures

References
-
- World Health Organization. GLOBOCAN Cancer Today: Prostate Cancer Fact Sheet. WHO 2018. Available online: http://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf
-
- World Health Organization. GLOBOCAN Cancer Today: China Fact Sheet. WHO 2018. Available online: https://gco.iarc.fr/today/data/factsheets/populations/160-china-fact-she...
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
