LncRNA-COX2 inhibits Fibroblast Activation and Epidural Fibrosis by Targeting EGR1
- PMID: 35280679
- PMCID: PMC8898373
- DOI: 10.7150/ijbs.67974
LncRNA-COX2 inhibits Fibroblast Activation and Epidural Fibrosis by Targeting EGR1
Abstract
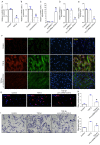
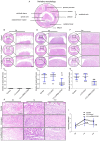
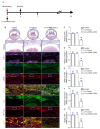
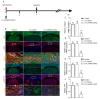
Rationale: Epidural fibrosis is one of the contributors to failed back surgery syndrome (FBSS) with a high incidence of about 80,000 cases per year. The fibrosis spreads from the operative region to the dura mater or the nerve root and results in functional incapacity and pain after laminectomy. Our previous study showed that down-regulation of lncRNA-COX2 is involved in the epidural scar formation. However, it remains unknown whether lncRNA-COX2 participate in the fibroblast activation and epidural fibrogenesis. Methods: LncRNA-COX2 and EGR1 expression were assessed by qRT-PCR and western blotting. Fibroblasts differentiation, proliferation and migration was determined by Collagen I/ɑ-SMA, 5-ethynyl-2'-deoxyuridine (EdU) and Transwell Assay respectively. Luciferase reporter assay was performed for the verification of target of LncRNA-COX2. Laminectomy was performed to establish the model of epidural fibrosis in mice. Epidural scar was evaluated by hematoxylin and eosin (HE) staining and Masson Trichrome staining. Results: Based on the result of transcriptome profiling, we found LncRNA-COX2 was significantly decreased in epidural tissues after laminectomy and in activated fibrotic fibroblasts. In vitro, overexpression of LncRNA-COX2 suppressed epidural fibrogenesis by inhibiting fibroblasts differentiation, proliferation and migration. Mechanistically, LncRNA-COX2 functioned as competing endogenous RNA (ceRNA) of EGR1. Gain of LncRNA-COX2 significantly decreased the expression of EGR1 and showed anti-fibrotic effect while EGR1 was markedly increased after loss of LncRNA-COX2. In vivo, LncRNA-COX2 attenuated laminectomy-induced epidural fibrosis in mice. Conclusion: In summary, the results demonstrated that LncRNA-COX2 showed anti-fibrotic effect by targeting EGR1 and identified LncRNA-COX2 as therapeutic molecule for preventing aberrant epidural fibrosis.
Keywords: EGR1.; Epidural Fibrosis; Fibroblast Activation; LncRNA-COX2; laminectomy.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Fritsch EW, Heisel J, Rupp S. The failed back surgery syndrome: reasons, intraoperative findings, and long-term results: a report of 182 operative treatments. Spine (Phila Pa 1976) 1996;21:626–33. - PubMed
-
- Amirdelfan K, Webster L, Poree L, Sukul V, McRoberts P. Treatment Options for Failed Back Surgery Syndrome Patients With Refractory Chronic Pain: An Evidence Based Approach. Spine (Phila Pa 1976) 2017;42(Suppl 14):S41–52. - PubMed
-
- Samy AM, Hardy RJ. Epidural fibrosis and the failed back surgery syndrome: history and physical findings. Neurol Res. 1999;21(Suppl 1):S5–8. - PubMed
-
- Zhang C, Kong X, Ning G, Liang Z, Qu T, Chen F, Cao D, Wang T, Sharma HS, Feng S. All-trans retinoic acid prevents epidural fibrosis through NF-kappaB signaling pathway in post-laminectomy rats. Neuropharmacology. 2014;79:275–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

