Caspase-1-mediated extracellular vesicles derived from pyroptotic alveolar macrophages promote inflammation in acute lung injury
- PMID: 35280692
- PMCID: PMC8898368
- DOI: 10.7150/ijbs.66477
Caspase-1-mediated extracellular vesicles derived from pyroptotic alveolar macrophages promote inflammation in acute lung injury
Abstract
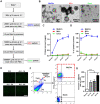
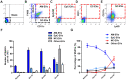
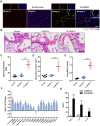
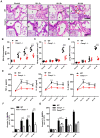
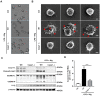
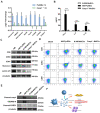
The occurrence and development of acute lung injury (ALI) involve a variety of pathological factors and complex mechanisms. How pulmonary cells communicate with each other and subsequently trigger an inflammatory cascade remains elusive. Extracellular vesicles (EVs) are a critical class of membrane-bound structures that have been widely investigated for their roles in pathophysiological processes, especially in immune responses and tumor progression. Most of the current knowledge of the functions of EVs is related to functions derived from viable cells (e.g., microvesicles and exosomes) or apoptotic cells (e.g., apoptotic bodies); however, there is limited understanding of the rapidly progressing inflammatory response in ALI. Herein, a comprehensive analysis of micron-sized EVs revealed a mass production of 1-5 μm pyroptotic bodies (PyrBDs) release in the early phase of ALI induced by lipopolysaccharide (LPS). Alveolar macrophages were the main source of PyrBDs in the early phase of ALI, and the formation and release of PyrBDs were dependent on caspase-1. Furthermore, PyrBDs promoted the activation of epithelial cells, induced vascular leakage and recruited neutrophils through delivery of damage-associated molecular patterns (DAMPs). Collectively, these findings suggest that PyrBDs are mainly released by macrophages in a caspase-1-dependent manner and serve as mediators of LPS-induced ALI.
Keywords: acute lung injury; alveolar macrophage; caspase-1; extracellular vesicles; pyroptosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. - PubMed
-
- McVey MJ, Steinberg BE, Goldenberg NM. Inflammasome Activation in Acute Lung Injury. Am J Physiol Lung Cell Mol Physiol. 2020. - PubMed
-
- Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E. et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. - PubMed
-
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A. et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

