Combined Detection of RUNX3 and EZH2 in Evaluating Efficacy of Neoadjuvant Therapy and Prognostic Value of Middle and Low Locally Advanced Rectal Cancer
- PMID: 35280723
- PMCID: PMC8907660
- DOI: 10.3389/fonc.2022.713335
Combined Detection of RUNX3 and EZH2 in Evaluating Efficacy of Neoadjuvant Therapy and Prognostic Value of Middle and Low Locally Advanced Rectal Cancer
Abstract
Objective: This article investigated whether Runt-Related Transcription Factor 3 (RUNX3) and enhancer of zeste homolog 2 (EZH2) can be used to evaluate the clinical efficacy of neoadjuvant therapy and prognosis of locally advanced rectal cancer (LARC).
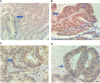
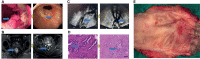
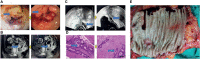
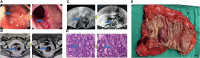
Methods: Eighty LARC patients admitted to the Tianjin Medical University Cancer Institute/Hospital and First Affiliated Hospital of Hebei North University from Jan 2015 to Jan 2016 were enrolled. The patients were followed up for 60 months through hospital visits. All patients received neoadjuvant chemoradiotherapy (long range radiotherapy + oral capecitabine) + total mesorecta excision (TME) surgery. The clinical efficacy of the treatments was evaluated through endoscopic, radiography, and tumor regression grade (TRG). In addition, expression level of RUNX3 and EZH2 was quantified via immunohistochemistry. The association of RUNX3 and EZH2 with clinicopathological characteristics of advanced tumors and efficacy of neoadjuvant therapy was explored. Logistic regression analysis was performed to identify predictors of efficacy of neoadjuvant chemoradiotherapy. Survival curve was used to evaluate the impact of RUNX3 and EZH2 on the prognosis of LARC patients.
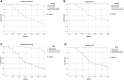
Results: A total of 80 patients diagnosed with LARC were enrolled in the study. Expression of RUNX3 was elevated in 25 (31.25%) patients, whereas expression of EZH2 was upregulated in 44 (55.00%) patients. Analysis of tumor regression identified 10 cases with TRG grade 0 (pathologic complete response, PCR), 24 cases with TRG grade 1, 35 cases with TRG grade 2, and 11 cases with TRG grade 3. Furthermore, 38 cases had significant down-staging, and 42 cases showed no significant down-staging as revealed by endoscopy and imaging. Patients with high expression of RUNX3 showed better tumor regression response and down-staging compared with those with low expression of RUNX3 (P < 0.001, P < 0.001). Moreover, patients with low EZH2 expression achieved TRG grade 0 and 1 response and down-staging effect compared with those with high expression of EZH2 (P < 0.001, P < 0.001). Logistic regression analysis showed that high expression of RUNX3, low expression of EZH2, and clinical N (cN) stage were good predictors of tumor regression response and down-staging. The 5-year disease free survival (DFS) and overall survival (OS) were 48.75 (39/80) and 58.75% (47/80), respectively. The 5-year DFS and OS of patients with high RUNX3 expression were significantly higher than low RUNX3 expression, whereas the 5-year DFS and OS of patients with high EZH2 expression were significantly lower than low EZH2 expression (P < 0.001). Univariate survival analysis showed that RUNX3 expression, EZH2 expression, cN, clinical T (cT), pathological T (pT) and pathological N (pN) were significantly correlated with the 5-year DFS and 5-year OS. Multivariate survival analysis showed that EZH2 expression and PN were good predictors of 5-year DFS and 5-year OS, whereas RUNX3 was a good predictor of 5-year DFS but not 5-year OS.
Conclusions: Expression level of RUNX3 and EZH2 accurately predicts clinical efficacy of neoadjuvant chemoradiotherapy and the prognosis of LARC patients, suggesting that RUNX3 and EZH2 can be used as pivotal clinical predictors for LARC.
Keywords: Runt-related transcription factor 3; histone-lysine N-methyltransferase EZH2; middle and low locally advanced rectal cancer; neoadjuvant therapy; prognosis; retrospective study.
Copyright © 2022 Wang, Wu, Xu, Gao, Wang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Sun M, Liu X, Xia L, Chen Y, Kuang L, Gu X, et al. A nine-lncRNA Signature Predicts Distant Relapse-Free Survival of HER2-Negative Breast Cancer Patients Receiving Taxane and Anthracycline-Based Neoadjuvant Chemotherapy. Biochem Pharmacol (2021) 189:114285. doi: 10.1016/j.bcp.2020.114285 - DOI - PubMed
LinkOut - more resources
Full Text Sources

