Adjuvant Therapy With PD1/PDL1 Inhibitors for Human Cancers: A Systematic Review and Meta-Analysis
- PMID: 35280727
- PMCID: PMC8913885
- DOI: 10.3389/fonc.2022.732814
Adjuvant Therapy With PD1/PDL1 Inhibitors for Human Cancers: A Systematic Review and Meta-Analysis
Abstract
Background: Immune checkpoint inhibitors (ICIs) have made a breakthrough in the systemic treatment of patients with advanced tumors. However, little is known about their efficacy and safety in adjuvant settings after the resection of solid tumors.
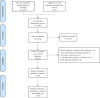
Methods: We performed a meta-analysis on the efficacy and safety of programmed death 1 (PD1)/PD-1 ligand (PDL1) inhibitors in adjuvant therapy after tumor resection using Review Manager 5.3, based on published clinical studies. The outcomes included recurrence-free survival (RFS), disease-free survival (DFS), overall survival (OS), and adverse events (AEs).
Results: Eight randomized controlled trials (RCTs) were included in the analysis. The use of PD1/PDL1 inhibitors in adjuvant therapy significantly improved RFS (hazard ratio [HR] = 0.72; 95% confidence interval [CI] 0.67-0.78, p < 0.00001). However, there was no statistically significant difference in OS between PD1/PDL1 inhibitors and placebo (HR = 0.86; 95% CI 0.74-1.00, p = 0.05). Gender, age, and PDL1 status were independent predictors of RFS with PD1/PDL1 inhibitors. As for the safety analysis results, PD1/PDL1 inhibitors had a higher incidence of fatigue (risk ratio [RR] = 1.22; 95% CI 1.01-1.49, p = 0.04), nausea (RR = 1.47; 95% CI 1.11-1.94, p = 0.007), and pruritus (RR = 1.96; 95% CI 1.57-2.44, p < 0.00001). In addition, the incidence of any grade adverse events increased in the PD1/PDL1 inhibitor group (RR = 1.03; 95% CI 1.02-1.05, p < 0.0001).
Conclusions: This is the first meta-analysis on the efficacy and safety of PD1/PDL1 inhibitors in adjuvant therapy. The use of PD1/PDL1 inhibitors in adjuvant therapy could significantly reduce the recurrence rate after solid tumor resection. However, the incidence of fatigue, nausea, pruritus, and any grade AEs also increased, which should be monitored with vigilance.
Keywords: PD1; PDL1; adjuvant therapy; human cancers; immune checkpoint inhibitor; meta-analysis.
Copyright © 2022 Jin, Wei, Weng, Feng, Xu, Wang, Cui, Chen, Wang and Peng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


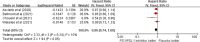


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

