An Up-To-Date Investigation Into the Correlation Between Proton Pump Inhibitor Use and the Clinical Efficacy of Immune Checkpoint Inhibitors in Advanced Solid Cancers: A Systematic Review and Meta-Analysis
- PMID: 35280736
- PMCID: PMC8907621
- DOI: 10.3389/fonc.2022.753234
An Up-To-Date Investigation Into the Correlation Between Proton Pump Inhibitor Use and the Clinical Efficacy of Immune Checkpoint Inhibitors in Advanced Solid Cancers: A Systematic Review and Meta-Analysis
Abstract
Background: Although immune checkpoint inhibitors (ICIs) have revolutionized the current anticancer therapies, a considerable proportion of patients are found to hardly benefit from these drugs. Accumulating studies have demonstrated that concomitant proton pump inhibitor (PPI) use may affect the clinical efficacy of ICIs; however, their results are inconsistent. In this study, based on updated evidence, we aimed to perform a meta-analysis to clarify the prognostic significance of PPI use in advanced solid cancer patients receiving ICI therapy.
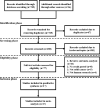
Methods: Eligible literature was searched using PubMed, Cochrane Library, Web of Science, EMBASE, and other network resources before July 2021. Clinical outcome was evaluated using overall survival (OS) and progression-free survival (PFS). The correlation of PPI use with OS or PFS was determined based on hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: A total of 17 studies enrolling 9,978 ICI-treated cancer patients were included in our meta-analysis. The global analysis demonstrated that PPI use was significantly correlated with worse OS [HR = 1.29 (1.10-1.50)] instead of PFS [HR = 1.19 (0.98-1.44)] in solid cancer patients receiving ICI therapy. In a subgroup analysis, the negative correlation of PPI use with ICI efficacy was significant in patients with non-small cell lung cancer [PFS, HR = 1.27 (1.10-1.47)] and urothelial carcinoma [OS, HR = 1.55 (1.31-1.84), PFS, HR = 1.52 (1.13-2.06)] and mixed cohorts containing multiple cancer types [OS, HR = 1.40 (1.16-1.69)], while an opposite result was observed in the PFS of patients with melanoma [HR = 0.48 (0.25-0.90)]. Moreover, the unfavorable prognostic impact of PPI use was also significant in patients over 65 years old [OS, HR = 1.28 (1.05-1.55), PFS, HR = 1.32 (1.12-1.56)] or those receiving anti-PD-1 [OS, HR = 1.37 (1.04-1.79)] or anti-PD-L1 therapies (OS, HR = 1.49 (1.30-1.69), PFS, HR = 1.34 (1.20-1.50). Finally, PPI use was significantly correlated with a worse prognosis in patients receiving PPIs 30 days before and/or after ICI initiation (OS, HR = 1.38 (1.18-1.62), PFS, HR = 1.23 (1.06-1.43)).
Conclusion: Although our global analysis revealed PPI use was not correlated with the PFS of ICI-treated patients, considering the results of our subgroup analysis, PPIs should be still cautiously used shortly before or during ICI therapy. Furthermore, more clinical validations and related mechanism investigations are of great necessity to clarify the clinical correlation of PPI use with ICI efficacy.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/], PROSPERO [No. CRD42021243707].
Keywords: cancer; immune checkpoint inhibitors; meta-analysis; prognosis; proton pump inhibitors.
Copyright © 2022 Liu, Guo, Mao, Tong, Yang and Yan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Yang M, Wang Y, Yuan M, Tao M, Kong C, Li H, et al. Antibiotic Administration Shortly Before or After Immunotherapy Initiation is Correlated With Poor Prognosis in Solid Cancer Patients: An Up-to-Date Systematic Review and Meta-Analysis. Int Immunopharmacol (2020) 88:106876. doi: 10.1016/j.intimp.2020.106876 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

