Clinical Benefit With PARP Inhibitor for Pathogenic Germline FANCA-Mutated Relapsed Epithelial Ovarian Cancer: A Case Report
- PMID: 35280757
- PMCID: PMC8913585
- DOI: 10.3389/fonc.2022.778545
Clinical Benefit With PARP Inhibitor for Pathogenic Germline FANCA-Mutated Relapsed Epithelial Ovarian Cancer: A Case Report
Abstract
Background: PARP inhibitors have been approved as targeted therapy for BRCA-deficient metastatic ovarian cancer (OC). Fanconi anemia complementation group A (FANCA), one of the homologous recombination repair pathway genes, is a susceptibility gene to breast cancer and OC. Therefore, it is interesting to investigate whether germline FANCA-mutated relapsed epithelial OC could achieve clinical benefit from the treatment of PARP inhibitor.
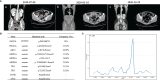
Case presentation: A 49-year-old female patient without a family history of cancer was diagnosed with epithelial OC. This patient underwent surgical resection plus platinum-based treatment twice in 2016 and 2018, successively. After the second relapse in July 2019, the patient underwent another radical resection. The next-generation sequencing analysis results revealed a germline FANCA mutation in the tumor tissue. Subsequently, the third-line treatment of liposomal doxorubicin hydrochloride plus lobaplatin was administrated for five cycles with the patient's consent. Then, oral niraparib (200 mg daily) was given for maintenance treatment. During the follow-up, no evidence of tumor recurrence was observed. Currently, the survival with no evidence of disease has already exceeded 21 months, and the treatment is still going on.
Conclusions: This case highlighted that OC patients harboring pathogenic gene alterations in the homologous recombination pathway might achieve clinical benefit from PARP inhibitors, which should be confirmed in further studies.
Keywords: FANCA; PARP inhibitor; clinical benefit; germline; ovarian cancer.
Copyright © 2022 Qian, Leng, Yan, Lu, Chen, Yi and Jiang.
Conflict of interest statement
ZY, SC, and YH were employed by the company 3D Medicines Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

