Altered Differentiation and Inflammation Profiles Contribute to Enhanced Innate Responses in Severe COPD Epithelium to Rhinovirus Infection
- PMID: 35280870
- PMCID: PMC8916560
- DOI: 10.3389/fmed.2022.741989
Altered Differentiation and Inflammation Profiles Contribute to Enhanced Innate Responses in Severe COPD Epithelium to Rhinovirus Infection
Abstract
Background: Respiratory viral infections are closely associated with COPD exacerbations, hospitalisations, and significant morbidity and mortality. The consequences of the persisting inflammation and differentiation status in virus associated severe disease is not fully understood. The aim of this study was to evaluate barrier function, cellular architecture, the inflammatory response in severe COPD bronchial epithelium to human rhinovirus (HRV) induced pathological changes and innate immune responses.
Methods: Well-differentiated primary bronchial epithelial cells (WD-PBECs) derived from severe COPD patients and age-matched healthy controls were cultured in the air-liquid interface (ALI) model. The differentiation phenotype, epithelial barrier integrity, pathological response and cytokine secreting profile of these cultures before and after HRV infection were investigated.
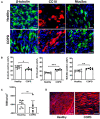
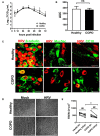
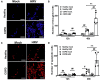
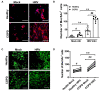
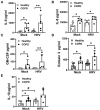
Results: WD-PBECs derived from severe COPD patients showed aberrant epithelium differentiation with a decreased proportion of ciliated cells but increased numbers of club cells and goblet cells compared with healthy controls. Tight junction integrity was compromised in both cultures following HRV infection, with heightened disruptions in COPD cultures. HRV induced increased epithelial cell sloughing, apoptosis and mucus hypersecretion in COPD cultures compared with healthy controls. A Th1/Th2 imbalance and a strong interferon and pro-inflammatory cytokine response was also observed in COPD cultures, characterized by increased levels of IFNγ, IFNβ, IP-10, IL-10 and decreased TSLP and IL-13 cytokine levels prior to HRV infection. Significantly enhanced basolateral secretion of eotaxin 3, IL-6, IL-8, GM-CSF were also observed in both mock and HRV infected COPD cultures compared with corresponding healthy controls. In response to HRV infection, all cultures displayed elevated levels of IFNλ1 (IL-29), IP-10 and TNFα compared with mock infected cultures. Interestingly, HRV infection dramatically reduced IFNλ levels in COPD cultures compared with healthy subjects.
Conclusion: An altered differentiation phenotype and cytokine response as seen in severe COPD WD-PBECs may contribute to increased disease susceptibility and an enhanced inflammatory response to HRV infection.
Keywords: ALI culture; COPD; bronchial epithelial cells; differentiation; host innate response; human rhinovirus.
Copyright © 2022 Guo-Parke, Linden, Mousnier, Scott, Killick, Borthwick, Fisher, Weldon, Taggart and Kidney.
Conflict of interest statement
IS and HK are employed by AstraZeneca. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

