Comparison of Clinical Characteristics and Short-Term Prognoses Within Hospitalized Chronic Obstructive Pulmonary Disease Patients Comorbid With Asthma, Bronchiectasis, and Their Overlaps: Findings From the ACURE Registry
- PMID: 35280888
- PMCID: PMC8914031
- DOI: 10.3389/fmed.2022.817048
Comparison of Clinical Characteristics and Short-Term Prognoses Within Hospitalized Chronic Obstructive Pulmonary Disease Patients Comorbid With Asthma, Bronchiectasis, and Their Overlaps: Findings From the ACURE Registry
Abstract
Introduction: Real-world evidence and comparison among commonly seen chronic obstructive pulmonary disease (COPD) phenotypes, i.e., asthma-COPD overlap (ACO), bronchiectasis-COPD overlap (BCO), and their coexistence (ABCO) have not been fully depicted, especially in Chinese patients.
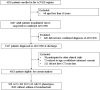
Methods: Data were retrieved from an ongoing nationwide registry in hospitalized patients due to acute exacerbation of COPD in China (ACURE).
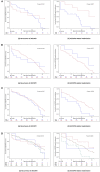
Results: Of the eligible 4,813 patients with COPD, 338 (7.02%), 492 (10.22%), and 63 (1.31%) were identified as ACO, BCO, and ABCO phenotypes, respectively. Relatively, the ABCO phenotype had a younger age with a median of 62.99 years [interquartile range (IQR): 55.93-69.48] and the COPD phenotype had an older age with a median of 70.15 years (IQR: 64.37-76.82). The BCO and COPD phenotypes were similar in body mass index with a median of 21.79 kg/m2 (IQR: 19.47-23.97) and 21.79 kg/m2 (IQR: 19.49-24.22), respectively. The COPD phenotype had more male gender (79.90%) and smokers (71.12%) with a longer history of smoking (median: 32.45 years, IQR: 0.00-43.91). The ACO and ABCO phenotypes suffered more prior allergic episodes with a proportion of 18.05 and 19.05%, respectively. The ACO phenotype exhibited a higher level of eosinophil and better lung reversibility. Moreover, the four phenotypes showed no significant difference neither in all-cause mortality, intensive care unit admission, length of hospital stay, and COPD Assessment Test score change during the index hospitalization, and nor in the day 30 outcomes, i.e., all-cause mortality, recurrence of exacerbation, all-cause, and exacerbation-related readmission.
Conclusions: The ACO, BCO, ABCO, and COPD phenotypes exhibited distinct clinical features but had no varied short-term prognoses. Further validation in a larger sample is warranted.
Keywords: asthma; bronchiectasis; chronic obstructive pulmonary disease; exacerbation; heterogeneity; phenotype; prognosis.
Copyright © 2022 Lei, Yang, Liang, Huang, Wu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Identification of Asthma-COPD Overlap, Asthma, and Chronic Obstructive Pulmonary Disease Phenotypes in Patients with Airway Obstruction: Influence on Treatment Approach.Respiration. 2020;99(1):35-42. doi: 10.1159/000503328. Epub 2019 Nov 6. Respiration. 2020. PMID: 31694032
-
Chronic obstructive pulmonary disease - diagnosis and management of stable disease; a personalized approach to care, using the treatable traits concept based on clinical phenotypes. Position paper of the Czech Pneumological and Phthisiological Society.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020 Dec;164(4):325-356. doi: 10.5507/bp.2020.056. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020. PMID: 33325455
-
Association between asthma/chronic obstructive pulmonary disease overlap syndrome and healthcare utilization among the US adult population.Curr Med Res Opin. 2019 Jul;35(7):1191-1196. doi: 10.1080/03007995.2019.1565531. Epub 2019 Jan 24. Curr Med Res Opin. 2019. PMID: 30612470
-
Characteristics, Management and In-Hospital Clinical Outcomes Among Inpatients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease in China: Results from the Phase I Data of ACURE Study.Int J Chron Obstruct Pulmon Dis. 2021 Feb 25;16:451-465. doi: 10.2147/COPD.S281957. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 33658775 Free PMC article. Review.
-
Current Knowledge of Asthma-COPD Overlap (ACO) Genetic Risk Factors, Characteristics, and Prognosis.COPD. 2021 Oct;18(5):585-595. doi: 10.1080/15412555.2021.1980870. Epub 2021 Sep 24. COPD. 2021. PMID: 34555990 Review.
Cited by
-
Clinical Benefits of Targeting Treatable Traits in Asthma and Chronic Obstructive Pulmonary Disease.Intern Med. 2025 Jan 1;64(1):17-23. doi: 10.2169/internalmedicine.3353-23. Epub 2024 Mar 4. Intern Med. 2025. PMID: 38432980 Free PMC article. Review.
-
Clinical Indicators for Asthma-COPD Overlap: A Systematic Review and Meta-Analysis.Int J Chron Obstruct Pulmon Dis. 2022 Oct 12;17:2567-2575. doi: 10.2147/COPD.S374079. eCollection 2022. Int J Chron Obstruct Pulmon Dis. 2022. PMID: 36259043 Free PMC article.
References
LinkOut - more resources
Full Text Sources

