Intensified Antituberculosis Therapy Regimen Containing Higher Dose Rifampin for Tuberculous Meningitis: A Systematic Review and Meta-Analysis
- PMID: 35280900
- PMCID: PMC8916538
- DOI: 10.3389/fmed.2022.822201
Intensified Antituberculosis Therapy Regimen Containing Higher Dose Rifampin for Tuberculous Meningitis: A Systematic Review and Meta-Analysis
Abstract
Background: Tuberculous meningitis is difficult to diagnose and is associated with high mortality. Recently, several studies evaluated the intensified regimen containing higher dose rifampin to treat tuberculous meningitis. However, this topic remains to be concluded. Therefore, this systematic review and meta-analysis was conducted to evaluate pharmacokinetics parameters, safety, and survival benefits of high-dose rifampin for tuberculous meningitis.
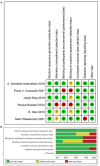
Method: Data were searched from PubMed, EMBASE, The Cochrane Library, and Web of Science for studies describing an antituberculosis regimen including a higher dose of rifampin for patients with tuberculous meningitis. The quality of eligible studies was evaluated via The Cochrane Risk of Bias Tool. The meta-analysis was performed by Review Manager 5.3 software, the synthesis of the data was shown in mean difference (MD) or relative risk (RR), and 95% confidence intervals (CIs).
Results: There were six randomized control trails included in this meta-analysis. The results showed that the concentration in plasma and cerebrospinal fluid (CSF) were significantly higher in the intervention group than the standard group [MD = 22.08, 95%CI (16.24, 27.92), p < 0.00001; MD = 0.74, 95%CI (0.42, 1.05), p < 0.00001], as well as the area under the time concentration curve between 0 and 24 h (AUC0-24) of rifampin [MD 203.56, 95%CI (153.07, 254.05), p < 0.00001] in plasma, but the overall survival did not improve [RR = 0.92, 95%CI (0.67, 1.26), p = 0.61]. For adverse events, the results showed a statistically significant lower incidence of hypersensitivity compared with the intervention group [RR = 1.72, 95%CI (1.13, 2.62), p = 0.01]. Fortunately, other common adverse drug reactions such as liver injury, neurological events, myelosuppression, and cardiotoxicity had no significant increase [RR = 0.98, 95%CI (0.77, 1.26), p = 0.90; RR = 1.10, 95%CI (0.94, 1.30), p = 0.23; RR = 0.82, 95%CI (0.59, 1.13), p = 0.22; RR = 1.11, 95%CI (0.66, 1.86), p = 0.70].
Conclusion: This meta-analysis suggested that the intensified treatment regimen including a higher dose of rifampin significantly increased the rifampin concentration both in the plasma and CSF, and it was safe in patients with tuberculous meningitis, but resulted in no improvement in survival rates.
Keywords: high-dose rifampin; meta-analysis; pharmacokinetics parameters; survival; tuberculous meningitis.
Copyright © 2022 Zhang, Wang and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




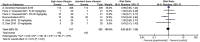

Similar articles
-
Fluoroquinolones in the management of tuberculous meningitis: Systematic review and meta-analysis.J Infect. 2018 Oct;77(4):261-275. doi: 10.1016/j.jinf.2018.06.009. Epub 2018 Jul 12. J Infect. 2018. PMID: 30017610
-
Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial.Lancet Infect Dis. 2013 Jan;13(1):27-35. doi: 10.1016/S1473-3099(12)70264-5. Epub 2012 Oct 25. Lancet Infect Dis. 2013. PMID: 23103177 Clinical Trial.
-
Intensified treatment with high dose rifampicin and levofloxacin compared to standard treatment for adult patients with tuberculous meningitis (TBM-IT): protocol for a randomized controlled trial.Trials. 2011 Feb 2;12:25. doi: 10.1186/1745-6215-12-25. Trials. 2011. PMID: 21288325 Free PMC article. Clinical Trial.
-
Safety and efficacy of high-dose rifampicin in the management of tuberculosis meningitis: Systematic review and meta-analysis.Int J Mycobacteriol. 2021 Jul-Sep;10(3):312-319. doi: 10.4103/ijmy.ijmy_135_21. Int J Mycobacteriol. 2021. PMID: 34494572
-
Clinical Outcomes of Patients With Drug-Resistant Tuberculous Meningitis Treated With an Intensified Antituberculosis Regimen.Clin Infect Dis. 2017 Jul 1;65(1):20-28. doi: 10.1093/cid/cix230. Clin Infect Dis. 2017. PMID: 28472255 Free PMC article. Clinical Trial.
Cited by
-
Mathematical Model for Growth and Rifampicin-Dependent Killing Kinetics of Escherichia coli Cells.ACS Omega. 2023 Oct 5;8(41):38452-38458. doi: 10.1021/acsomega.3c05233. eCollection 2023 Oct 17. ACS Omega. 2023. PMID: 37867679 Free PMC article.
-
Extreme and Severe Systemic Pain Caused by Rifampicin: A Case Report of a Rare Allergic Reaction.Curr Drug Saf. 2025;20(4):539-544. doi: 10.2174/0115748863359479250121114135. Curr Drug Saf. 2025. PMID: 39931993
References
-
- World Health Organization . Global Tuberculosis Report (2020).
-
- WHO Guidelines Approved by the Guidelines Review Committee . Treatment of Tuberculosis: Guidelines. Geneva: World Health Organization; (2010).
Publication types
LinkOut - more resources
Full Text Sources

