The Research Progress of Antiangiogenic Therapy, Immune Therapy and Tumor Microenvironment
- PMID: 35281003
- PMCID: PMC8905241
- DOI: 10.3389/fimmu.2022.802846
The Research Progress of Antiangiogenic Therapy, Immune Therapy and Tumor Microenvironment
Abstract
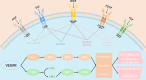
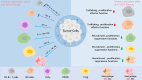
Anti-angiogenesis therapy, a promising strategy against cancer progression, is limited by drug-resistance, which could be attributed to changes within the tumor microenvironment. Studies have increasingly shown that combining anti-angiogenesis drugs with immunotherapy synergistically inhibits tumor growth and progression. Combination of anti-angiogenesis therapy and immunotherapy are well-established therapeutic options among solid tumors, such as non-small cell lung cancer, hepatic cell carcinoma, and renal cell carcinoma. However, this combination has achieved an unsatisfactory effect among some tumors, such as breast cancer, glioblastoma, and pancreatic ductal adenocarcinoma. Therefore, resistance to anti-angiogenesis agents, as well as a lack of biomarkers, remains a challenge. In this review, the current anti-angiogenesis therapies and corresponding drug-resistance, the relationship between tumor microenvironment and immunotherapy, and the latest progress on the combination of both therapeutic modalities are discussed. The aim of this review is to discuss whether the combination of anti-angiogenesis therapy and immunotherapy can exert synergistic antitumor effects, which can provide a basis to exploring new targets and developing more advanced strategies.
Keywords: antiangiogenic therapy; cancer biology; immune therapy; progress; tumor microenvironment.
Copyright © 2022 Hu, Chen, Tan, Wu, Huang, Fu, Luo and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Soria J-C, Mauguen A, Reck M, Sandler A, Saijo N, Johnson D, et al. Systematic Review and Meta-Analysis of Randomised, Phase Ii/Iii Trials Adding Bevacizumab to Platinum-Based Chemotherapy as First-Line Treatment in Patients With Advanced Non-Small-Cell Lung Cancer. Ann Oncol (2012) 24(1):20–30. doi: 10.1093/annonc/mds590 - DOI - PubMed
-
- Reck M, Kaiser R, Mellemgaard A, Douillard J, Orlov S, Krzakowski M, et al. Docetaxel Plus Nintedanib Versus Docetaxel Plus Placebo in Patients With Previously Treated Non-Small-Cell Lung Cancer (Lume-Lung 1): A Phase 3, Double-Blind, Randomised Controlled Trial. Lancet Oncol (2014) 15(2):143–55. doi: 10.1016/s1470-2045(13)70586-2 - DOI - PubMed
-
- Garon EB, Ciuleanu T-E, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab Plus Docetaxel Versus Placebo Plus Docetaxel for Second-Line Treatment of Stage Iv Non-Small-Cell Lung Cancer After Disease Progression on Platinum-Based Therapy (Revel): A Multicentre, Double-Blind, Randomised Phase 3 Trial. Lancet (2014) 384(9944):665–73. doi: 10.1016/S0140-6736(14)60845-X - DOI - PubMed
-
- Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, et al. Gemcitabine Plus Bevacizumab Compared With Gemcitabine Plus Placebo in Patients With Advanced Pancreatic Cancer: Phase III Trial of the Cancer and Leukemia Group B (Calgb 80303). J Clin Oncol (2010) 28(22):3617. doi: 10.1200/JCO.2010.28.1386 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

