Multi-Cellular Immunological Interactions Associated With COVID-19 Infections
- PMID: 35281033
- PMCID: PMC8913044
- DOI: 10.3389/fimmu.2022.794006
Multi-Cellular Immunological Interactions Associated With COVID-19 Infections
Abstract
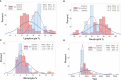
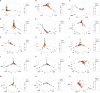
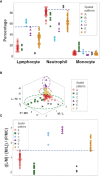
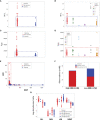
To rapidly prognosticate and generate hypotheses on pathogenesis, leukocyte multi-cellularity was evaluated in SARS-CoV-2 infected patients treated in India or the United States (152 individuals, 384 temporal observations). Within hospital (<90-day) death or discharge were retrospectively predicted based on the admission complete blood cell counts (CBC). Two methods were applied: (i) a "reductionist" one, which analyzes each cell type separately, and (ii) a "non-reductionist" method, which estimates multi-cellularity. The second approach uses a proprietary software package that detects distinct data patterns generated by complex and hypothetical indicators and reveals each data pattern's immunological content and associated outcome(s). In the Indian population, the analysis of isolated cell types did not separate survivors from non-survivors. In contrast, multi-cellular data patterns differentiated six groups of patients, including, in two groups, 95.5% of all survivors. Some data structures revealed one data point-wide line of observations, which informed at a personalized level and identified 97.8% of all non-survivors. Discovery was also fostered: some non-survivors were characterized by low monocyte/lymphocyte ratio levels. When both populations were analyzed with the non-reductionist method, they displayed results that suggested survivors and non-survivors differed immunologically as early as hospitalization day 1.
Keywords: COVID-19; biological complexity; cutoff-free; error prevention; multi-cellularity; pattern recognition; personalized medicine; personalized methods.
Copyright © 2022 Verma, Libertin, Gupta, Khanna, Kumar, Arora, Krishna, Fasina, Hittner, Antoniades, van Regenmortel, Durvasula, Kempaiah and Rivas.
Conflict of interest statement
Author AA was employed by the company Stremble Ventures, LTD. Author AR is a co-inventor of the temporary guides used to recognize data patterns (European Union patent number 2959295, US patent number 10,429,389 B2). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures











References
-
- Auffray C, Noble D, Nottale L, Turner P. Progress in Integrative Systems Biology, Physiology and Medicine: Towards a Scale-Relative Biology. Eur Phys J A (2020) 56:88. doi: 10.1140/epja/s10050-020-00090-3 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

