Functional Roles of Chemokine Receptor CCR2 and Its Ligands in Liver Disease
- PMID: 35281057
- PMCID: PMC8913720
- DOI: 10.3389/fimmu.2022.812431
Functional Roles of Chemokine Receptor CCR2 and Its Ligands in Liver Disease
Abstract
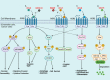
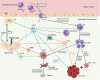
Chemokines are a family of cytokines that orchestrate the migration and positioning of immune cells within tissues and are critical for the function of the immune system. CCR2 participates in liver pathology, including acute liver injury, chronic hepatitis, fibrosis/cirrhosis, and tumor progression, by mediating the recruitment of immune cells to inflammation and tumor sites. Although a variety of chemokines have been well studied in various diseases, there is no comprehensive review presenting the roles of all known chemokine ligands of CCR2 (CCL2, CCL7, CCL8, CCL12, CCL13, CCL16, and PSMP) in liver disease, and this review aims to fill this gap. The introduction of each chemokine includes its discovery, its corresponding chemotactic receptors, physiological functions and roles in inflammation and tumors, and its impact on different immune cell subgroups.
Keywords: CCL2; CCR2; PSMP; chemokine; hepatocellular carcinoma; macrophage.
Copyright © 2022 She, Ren, Chen, Wang, Chen, Wang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, et al. . International Union of Basic and Clinical Pharmacology. [Corrected]. LXXXIX. Update on the Extended Family of Chemokine Receptors and Introducing a New Nomenclature for Atypical Chemokine Receptors. Pharmacol Rev (2013) 66:1–79. doi: 10.1124/pr.113.007724 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

