A Thermostable Oral SARS-CoV-2 Vaccine Induces Mucosal and Protective Immunity
- PMID: 35281065
- PMCID: PMC8913903
- DOI: 10.3389/fimmu.2022.837443
A Thermostable Oral SARS-CoV-2 Vaccine Induces Mucosal and Protective Immunity
Abstract
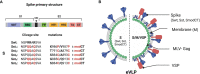
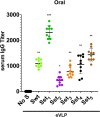
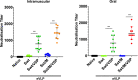
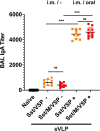
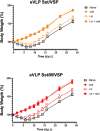
An ideal protective vaccine against SARS-CoV-2 should not only be effective in preventing disease, but also in preventing virus transmission. It should also be well accepted by the population and have a simple logistic chain. To fulfill these criteria, we developed a thermostable, orally administered vaccine that can induce a robust mucosal neutralizing immune response. We used our platform based on retrovirus-derived enveloped virus-like particles (eVLPs) harnessed with variable surface proteins (VSPs) from the intestinal parasite Giardia lamblia, affording them resistance to degradation and the triggering of robust mucosal cellular and antibody immune responses after oral administration. We made eVLPs expressing various forms of the SARS-CoV-2 Spike protein (S), with or without membrane protein (M) expression. We found that prime-boost administration of VSP-decorated eVLPs expressing a pre-fusion stabilized form of S and M triggers robust mucosal responses against SARS-CoV-2 in mice and hamsters, which translate into complete protection from a viral challenge. Moreover, they dramatically boosted the IgA mucosal response of intramuscularly injected vaccines. We conclude that our thermostable orally administered eVLP vaccine could be a valuable addition to the current arsenal against SARS-CoV-2, in a stand-alone prime-boost vaccination strategy or as a boost for existing vaccines.
Keywords: COVID-19; VLP (virus-like particle); mucosal immunity; oral vaccination; vaccine.
Copyright © 2022 Bellier, Saura, Luján, Molina, Luján and Klatzmann.
Conflict of interest statement
HL and DK are inventors of a patent application claiming orally administered vaccines against coronaviruses that is owned by their public institutions. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Sultana J, Mazzaglia G, Luxi N, Cancellieri A, Capuano A, Ferrajolo C, et al. . Potential Effects of Vaccinations on the Prevention of COVID-19: Rationale, Clinical Evidence, Risks, and Public Health Considerations. Expert Rev Vaccines (2020) 19:919–36. doi: 10.1080/14760584.2020.1825951 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

