Efferocytosis and Its Role in Inflammatory Disorders
- PMID: 35281078
- PMCID: PMC8913510
- DOI: 10.3389/fcell.2022.839248
Efferocytosis and Its Role in Inflammatory Disorders
Abstract
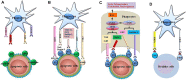
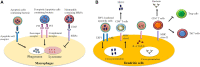
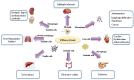
Efferocytosis is the effective clearance of apoptotic cells by professional and non-professional phagocytes. The process is mechanically different from other forms of phagocytosis and involves the localization, binding, internalization, and degradation of apoptotic cells. Defective efferocytosis has been demonstrated to associate with the pathogenesis of various inflammatory disorders. In the current review, we summarize recent findings with regard to efferocytosis networks and discuss the relationship between efferocytosis and different immune cell populations, as well as describe how efferocytosis helps resolve inflammatory response and modulate immune balance. Our knowledge so far about efferocytosis suggests that it may be a useful target in the treatment of numerous inflammatory diseases.
Keywords: apoptosis; efferocytosis; immune response; inflammatory diseases; phagocytosis.
Copyright © 2022 Ge, Huang and Yao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

