Toll-like Receptor 2 is Involved in Calcium Influx and Acrosome Reaction to Facilitate Sperm Penetration to Oocytes During in vitro Fertilization in Cattle
- PMID: 35281105
- PMCID: PMC8907135
- DOI: 10.3389/fcell.2022.810961
Toll-like Receptor 2 is Involved in Calcium Influx and Acrosome Reaction to Facilitate Sperm Penetration to Oocytes During in vitro Fertilization in Cattle
Abstract
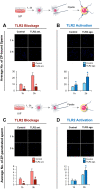
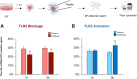
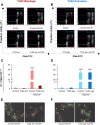
Cumulus cells of ovulated cumulus-oocyte complexes (COCs) express Toll-like receptor 2 (TLR2), pathogen recognition receptors, to recognize and react to sperm signals during fertilization. Sperm also express TLR2, but its contribution to the sperm-oocytes crosstalk is still unclear. Here, we adapted the in vitro fertilization (IVF) model to characterize the potential relevance of sperm TLR2 in sperm-oocytes interactions during fertilization in bovine. The IVF results showed that the ligation of sperm TLR2 with its specific antagonist/agonist resulted in down/up-regulation of the cleavage and blastocyst rates either in COCs or cumulus-free oocytes, but not in zona pellucida (ZP)-free oocytes. The computer-assisted sperm analysis (CASA) system revealed that sperm motility parameters were not affected in TLR2 antagonist/agonist-treated sperm. However, fluorescence imaging of sperm-ZP interactions revealed that the blockage or activation of the TLR2 system in sperm reduced or enhanced both binding and penetration abilities of sperm to ZP compared to control, respectively. Flow cytometrical analysis of acrosome reaction (AR) demonstrated that the TLR2 system adjusted the occurrence of AR in ZP-attached sperm, suggesting that sperm TLR2 plays physiological impacts on the sperm-oocyte crosstalk via regulating ZP-triggered AR in sperm. Given that calcium (Ca2+) influx is a pre-requisite step for the induction of AR, we investigated the impact of the TLR2 system on the ionophore A23187-induced Ca2+ influx into sperm. Notably, the exposure of sperm to TLR2 antagonist/agonist reduced/increased the intracellular Ca2+ level in sperm. Together, these findings shed new light that the TLR2 system is involved in sperm AR induction which enables sperm to penetrate and fertilize oocytes during the fertilization, at least in vitro, in cows. This suggests that sperm possibly developed a quite flexible sensing mechanism simultaneously against pathogens as well as COCs toward fertilization with the same TLR2 of the innate immune system.
Keywords: acrosome reaction; bovine; in vitro fertilization; intracellular Ca2+ influx; sperm; toll-like receptor 2.
Copyright © 2022 Ma, Marey, Shimada and Miyamoto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
A simple method for assessment of the human acrosome reaction of spermatozoa bound to the zona pellucida: lack of relationship with ionophore A23187-induced acrosome reaction.Hum Reprod. 1996 Mar;11(3):551-7. doi: 10.1093/humrep/11.3.551. Hum Reprod. 1996. PMID: 8671264
-
Inhibitors of serine proteases decrease sperm penetration during porcine fertilization in vitro by inhibiting sperm binding to the zona pellucida and acrosome reaction.Theriogenology. 2015 Nov;84(8):1378-86. doi: 10.1016/j.theriogenology.2015.07.022. Epub 2015 Jul 26. Theriogenology. 2015. PMID: 26303413
-
Fertilization with human sperm bound to zona pellucida by pressing onto the oocyte membrane.Hum Cell. 2020 Jul;33(3):521-527. doi: 10.1007/s13577-020-00348-4. Epub 2020 Mar 14. Hum Cell. 2020. PMID: 32172344
-
Dynamic regulation of sperm interactions with the zona pellucida prior to and after fertilisation.Reprod Fertil Dev. 2012;25(1):26-37. doi: 10.1071/RD12277. Reprod Fertil Dev. 2012. PMID: 23244826 Review.
-
Acrosome reaction in the cumulus oophorus revisited: involvement of a novel sperm-released factor NYD-SP8.Protein Cell. 2011 Feb;2(2):92-8. doi: 10.1007/s13238-011-1022-5. Epub 2011 Mar 5. Protein Cell. 2011. PMID: 21380641 Free PMC article. Review.
Cited by
-
Toll-like receptors in mammalian sperm.Reprod Med Biol. 2025 Apr 15;24(1):e12651. doi: 10.1002/rmb2.12651. eCollection 2025 Jan-Dec. Reprod Med Biol. 2025. PMID: 40242391 Free PMC article. Review.
-
TLR2 and TLR4 bridge physiological and pathological inflammation in the reproductive system.Commun Biol. 2025 Jul 5;8(1):1008. doi: 10.1038/s42003-025-08424-x. Commun Biol. 2025. PMID: 40618011 Free PMC article. Review.
-
Ultrastructural evidence for the activation of autophagy and analysis of the protective role of autophagy in goat spermatozoa under liquid storage.Front Vet Sci. 2025 Mar 13;12:1543459. doi: 10.3389/fvets.2025.1543459. eCollection 2025. Front Vet Sci. 2025. PMID: 40151572 Free PMC article.
-
Toll-like receptor 2 activation of early divided bovine embryo promotes its viability and development competence in vitro.Sci Rep. 2025 Jul 9;15(1):24678. doi: 10.1038/s41598-025-09570-2. Sci Rep. 2025. PMID: 40634450 Free PMC article.
-
A citrullinated histone H3 monoclonal antibody for immune modulation in sepsis.Nat Commun. 2025 Aug 12;16(1):7435. doi: 10.1038/s41467-025-62788-6. Nat Commun. 2025. PMID: 40796783 Free PMC article.
References
-
- Akthar I., Kim Y., Kanno C., Horiguchi S., Sasaki M., Marey M. A., et al. (2020). Toll-like receptor 2 of bull sperm and uterus co-regulates sperm sensing by uterine gland to trigger the innate immune responses. The 113th Society of Reproduction and Development Meeting, Sendai, Japan. 10.14882/jrds.113.0_P-27 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

